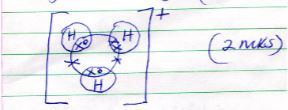CHEMISTRY
PAPER 1
(THEORY)
INSTRUCTIONS TO CANDIDATES
- Write your name, admission number in the spaces provided above
- Answer all the questions in the spaces provided
- KNEC Mathematical tables and silent electronic calculator may be used.
- All the working must be shown clearly where necessary
- Candidates should answer questions in English.
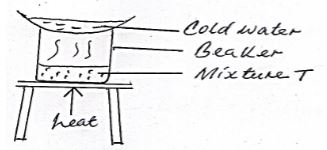
- The set up below was used to separate a mixture of two substances.
One of the components in the mixture T was sodium chloride.- Name the other component (1mk)
- Name the method of separation (1mk)
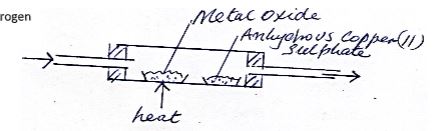
- Dry hydrogen gas was passed through a hot metal oxide as shown in the diagram below.
The metal oxide turned from black to brown.- . Identify the metal oxide (1mk)
- State one other observation in the combustion tube. (1mk)
- Write a chemical equation for one of the reaction taking place in the combustion tube. (1mk)
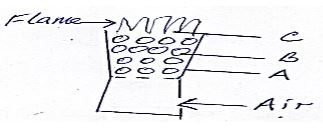
- The diagram below shows regions of a burning charcoal stove.
- Write an equation for the reaction taking place at
- B (1mk)
- C (1mk)
- Name the allotropes of carbon represented by the diagram below (2mks)
- Write an equation for the reaction taking place at
- Student are advised to use a non- luminous flame when heating during laboratory experiments.
- Why is the non-luminous flame preferred. (1mk)
- How does a Bunsen Burner produce a non-luminous flame. (1mk)
-
- State Boyles Law (1mk)
- A balloon contains 250dm3 of helium at 25˚c and 100kPa pressure. Calculate the temperature when its volume reaches 400dm3 and 80kpa pressure (2mks)
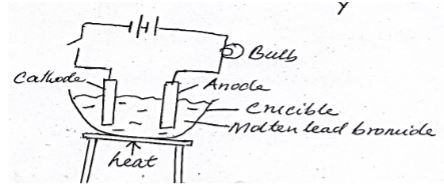
- Below is a diagram of a set up of apparatus used to investigate the effect of electric current on a binary electrolyte, lead (II) bromide.
- What is a binary electrolyte. (1mk)
- What is the importance of heating in the above experiment? (1mk)
- Write the ionic equation for the reaction taking place at the anode (1mk)
- Explain how the following factors affect the rate of a chemical reaction
- Temperature (2mks)
- Concentration (2mks)
- Study the reaction below and answer the questions that follows
2NO2g → N2O4(g) ΔH = -ve
Brown Pale yellow- State the observation made when a mixture of NO2 (g) and N2O4 (g) at equilibrium is subjected to
- High pressure (½mk)
- High temperatures (½mk)
- Explain the observations in a(i) and a(ii) (1mk)
- State the observation made when a mixture of NO2 (g) and N2O4 (g) at equilibrium is subjected to
- The set up below shows the catalytic oxidation of ammonia in the laboratory.
One of the observations made in the flask is that the platinum wire glows even though it is no longer heated.- Explain this observation (1mk)
- State another observation made in the conical flask (1mk)
- Write an equation to explain the second observation (1mk)
-
- Distinguish between a dative and covalent bond (2mks)
- Draw a diagram to show bonding in hydroxonium ion (H3O+) (H=1, O = 8) (2mks)
- The information below gives pH value of solution V, W, X, Y, Z
Solution pH Values V 2 W 6.5 X 11 Y 14 Z 4.5 - Which solution is likely to be
- Calcium hydroxide? (1mk)
- Rain Water (1mk)
- Explain the pH value of rain water (1mk)
- Which solution is likely to be
- 20cm3 of a dibasic acid required 25cm3 of 0.1M sodium hydroxide for complete neutralisation.
- How many moles of sodium hydroxide reacted with the dibasic acid ? (1mk)
- Calculate the concentration of the dibasic acid in moles per litre (2mks)
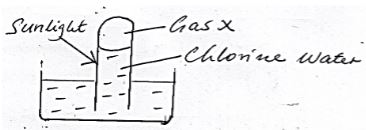
- Chloride water was exposed to sunlight as shown in the set up below x
- Write an equation for the reaction that produce gas X. (1mk)
- Explain the observation made if a red flower petal is immersed in chlorine water. (1mk)
- The table below shows the solubility of a substance at various temperatures. Study it and answer the questions that follows.
- What is the meaning of solubility (1mk)
- State and explain what would happen if a sample of a saturated solution of the substance at 40˚c was heated to 110˚c (1mk)
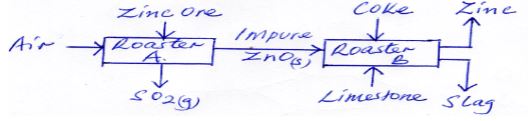
- The flow chart below shows the processes involved in extraction of Zinc metal. Study it and answer the questions that follow.
- Name the main ore used in the extraction of Zinc. (1mk)
- What is the function of limestone in roaster B (1mk)
- State one use of Zinc (1mk)
- The products formed by action of heat on nitrates of elements P,Q,R are shown in the table below.
Nitrate of element Products formed P
Q
RMetal Oxide + Nitrogen(iv) Oxide + Oxygen
Metal + Oxygen + Nitrogen (iv) Oxide
Metal Nitrite + Oxygen- Arrange the metal elements in order of increasing reactivity (1mk)
- Which element forms a soluble carbonate (1mk)
- Give an example of element Q (1mk)
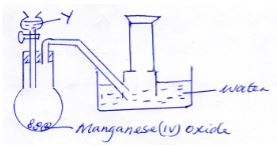
- A student set up the apparatus shown below to prepare and collect oxygen gas.
He made a mistake in the set and could not collect the gas.- What was the mistake (1mk)
- Identify substances Y (1mk)
- Write an equation for the reaction in the flask (1mk)
-
- A radioactive element weighed 384g. After 270days, its mass fell to 48g. Calculate the half -life of the element. (2mks)
- State one application of radioisotopes in industry. (1mk)
- Three portions of a solution of B(NO3)2 (B is not the actual symbol of the element) where tested as follows.
- When 2cm3 portions of dilute hydrochloric acid is added to the first portion, a white precipitate was formed which dissolve on warming.
- When two drops of 2M sodium hydroxide solution were added to the second portion, a white precipitate is formed which dissolved in excess sodium hydroxide to form a colourless solution.
- When a solution of potassium iodide was added to the third portion, a yellow precipitate was formed.
-
- Identify the
- White precipitate formed in step 1 (1mk)
- Element B (1mk)
- Write an ionic equation for the reaction that occurs in step (iii) (1mk)
- Identify the
- The following table gives standard electrode potentials for a number of half cell reactions.
Zn2+(aq) + 2e- → Zn(s) -0.70
Fe2+(aq) + 2e- → Fe(s) -0.44
I2 + 2e- → 2I-(aq) +0.54- Which of the substances listed above is the strongest oxidising agent. Explain (2mks)
- Write the cell representation that would give the highest e.m.f value (1mk)
- 0.6g of element K (not actual symbol) were completely burnt in oxygen to heat 500cm3 of water. Given that the RAM of X is 12.06, determine the final temperature of the water heated if the initial temperature was 21˚c. Specific heat capacity of water = 4.2Jk-1g
Density of water = 1.0gcm³
Molar heat of combustion of K is 380kJ mol (3mks)- Name two reagent that can be used to prepare sulphur (iv) oxide gas in the laboratory. (2mks)
- When a tube containing moist sulphur (iv) oxide gas is inverted over one containing hydrogen sulphide gas a yellow deposit forms on the sides of both tubes. Explain (2mks)
- State one use of sulphur (iv) oxide gas (1mk)
- A container in the lab has the label CH3CH2COOCH3.
- Name the process of formation of the above substance (1mk)
- Identify the two substances from which the substance is made (2mks)
- Name the condition necessary for the formation of CH3CH2COOCH3- (1mk)
- In an electrolytic process a current of 200A was passed through molten oxide of a metal Q for 58 minutes and 64.8g of the metal deposited. Determine
- Charge on metal Q (R.M.M of Q =27) (1mk)
- The volume of oxygen produced at standard temperature and pressure (IF= 96500c, Molar gas volume at StP = 22.4dm³) (2mks)
- Describe how a student can distinguish between Calcium and Lead ions in solution using aqueous sodium chloride. (2mks)
- The table below shows the 1st and 2nd ionization energies of three elements in the same family of the periodic table.
Element 1st ionisation energy kJ/mol 2nd Ionisation energy kJ/mol K 900 1880 L 736 1450 M 590 1150 - Arrange the above element in order of decreasing reactivity (1mk)
- Explain why the 1st ionization energy is higher than the second ionization energy (2mks)
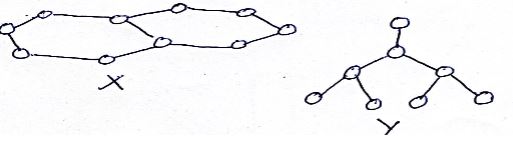
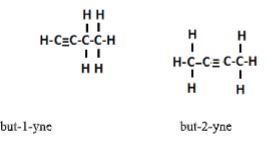
- Draw and name two isomers of C4H6 (3mks)
MARKING SCHEME
-
- Ammonium chloride/iodine/iron (ii) chloride (1mk)
- Sublimation (1mk)
-
- Copper (ii) oxide (1mk)
- Anhydrous copper (II) sulphate turn from white to blue. (1mk)
- CUO(s) + H2 → CU(s) + H2O(l) (1mk)
CUSO4(s) +5H2O(l) → CUSO4 5H2O(S) (1MK)
-
-
- CO2 + C → 2CO(g) (1mk)
- 2CO(g) + O29g) → 2CO29(g) (1mk)
- X – Graphite (1mk) Y – Diamond (1mk)
-
-
-
- It is hotter ½mk
- Does not form soot ½mk
- Bu opening air holes fully. (1mk)
-
-
- The volume of a fixed mass of gas is inversely proportional to pressure at constant temperature. (1mk)
- P1 V1/T1 = . P2 V2/T2 = T2 = . P2V2T1/P1 V1 ½mk
80 x 400x 298 1mk
100 x 250
T2 = 381.44K or 108.44˚c (½mks)
-
- An electrolyte made of two elements (1mk)
- To melt lead bromide so that the ions are free/mobile (1mk)
- 2Br(l) → Br2(g) + 2e- (1mk)
-
- Temperature – At high temperature more particles have enough kinetic energy. More effective collissions. (2mks)
- Concentration – more high concentration there are higher chances of effective collissions. (2mks)
-
- Mixture turns pale yellow(½mk) – equilibrium shifts to the right as (½mk )there are fewer molecules 1½mk
- Mixture turns brown(½mk) – equilibrium shift to the left (½mk) as backward reaction is endothermic
-
- Reaction is exothermic (1mk)
- Red brown fumes (1mk)
- 2NO(g) + O2(g) → 2NO2(g) (1mk)
-
- Dative bond – one of the atoms produce the shared pair of electrons. (1mk
Covalent bond – Each of the bonding atoms produce equal number of electrons for sharing (1mk) -
- Dative bond – one of the atoms produce the shared pair of electrons. (1mk
-
-
- X (1mk)
- W (1mk)
- Rain water dissolves carbon (iv) oxide to form weak carbonic acid. (1mk)
-
-
- 25/1000 (½mk) x 0.1 = 0.0025moles (½mk)
- Moles of dibasic acid 0.0025/2 (½mk) = 0.00125 (½mk)
Concentration 0.00125 x 1000/20 (½mk)
=0.0625 M (½mk)
- 25/1000 (½mk) x 0.1 = 0.0025moles (½mk)
-
- 2HOCL (aq) → O2 (g) + 2HCL(aq)
- The dye is bleached by chloric (i) acid in chlorine water (1mk)
HOCL + Dye Dye + O + HCL
-
- Maximum mass of solute that saturate 100g of water at a given temperature (1mk)
- Solution becomes less saturated // crystals of solute forms (1mk)
-
- Zinc blende (1mk)
- to remove silica impurities (1mk)
- Galvanisation, making dry cells. (1mk)
-
- Q P R (1mk)
- R (1mk)
- Silver/mercury (1mk)
-
- Beehive shelf should be below water level in the water tough (1mk)
- Hydrogen peroxide / H2O2 (1mk)
- 2H2O2 → 2H2O (l) + O2(g) (1mk)
-
- 384 → 192 → 96 → 48 (1mk)
No of half lifes = 3
Half-life = 270/3 = 90days (1mk) -
- Industrial – controlling thickness of sheets
- leakages in underground pipes
- atomic bombs
- any other
- 384 → 192 → 96 → 48 (1mk)
-
- Lead chloride PbCl2(1mk)
B- Pb (1mk) - Pb2+(aq) + 2I-(aq) → PbI (1mk)
- Lead chloride PbCl2(1mk)
-
- I2 – Has the lowest electrode potential // Most positive (2mks)
- Zn (s)/Zn2+(aq)//2I-9(aq) /I2 (1mk)
- I2 – Has the lowest electrode potential // Most positive (2mks)
- Moles of K = 0.6/12.06 = 0.04975 (1mk)
18.90 K = 500/1000 x 4.2 DT (½mk)
Heat change = 0.04975 x 380 = 18.905kJ (½mk)
18.90 = 2.1DT
DT = 18.90/2.1 (½mk)= 9
Final temperature = 21 + 9
= 30˚c (½mk) -
-
- Sodium sulphite and dil. Hydrochloric acid (2mks)
- Copper turning and conc. Sulphuric acid.
- Hydrogen sulphide gas is oxidised to sulphur while sulphur (iv) oxide is reduced to sulphur (2mks)
2H2S(g) + SO2(g) → 3S(s) + 2H2O(l) -
- Manufacture of sulphuric acid. (1mks)
- Fumigation
- As a preservative in fruit juices.
-
-
- Esterification (1mk)
- Methanol and propanoic acid. (2mks)
CH3OH CH3CH2COOH - Conc. Sulphuric (vi) acid catalyst (1mk)
-
- Q = It
= 200 x 58 x 60
= 696000 (½mk)
64.8 → 69600
27 - → 696000 x 27
64.8
= 290,000C
No. of faradays 29000096500 = 3 (½ mk)
Charge = +3 - 1 mole of O2 – 4 faradays (4 x 96500) ½mk)
22.4dm³ - 4 x 96500
? - 290,000
290000 4 x 96500 x 22.4 (1mk) = 16.8290dm³ (½mk)
- Q = It
-
- To each solution of ions add a few drops of sodium chloride.
- White precipitate forms in one containing lead ions. (1mk)
- No precipitate is formed in one with calcium ions. (1mk)
-
- M L K (1mk)
- Effective nuclear charge increases after removal of the first electron more energy is therefore required to remove the 2nd electron. (2mks)
-
Download Chemistry Paper 1 Questions and Answers - MECS Cluster Joint Mock Exams 2021/2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students