- Introduction
- Structure of the Membrane
- Properties of the cell membrane
- Physiological Processes of the Cell membrane
- Diffusion
- Osmosis
- Active Transport

Introduction
- Physiology refers to the branch of biology that deals with the study of functions and activities of life or of living matter such as organs, tissues or cells. It aims at understanding the mechanism of living.
- In simpler terms, physiology refers to the processes and functions that take place inside the body cells of organisms.
- Cell physiology refers to the study of functions of the cell structures. The cell structures perform various functions of life. In particular:
- Chloroplasts play a vital role in carbohydrate synthesis.
- Mitochondrion produces energy required to carry out life processes.
- Ribosomes manufacture of proteins.
- These physiological processes require various raw materials for them to take place.
- For photosynthesis to occur, carbon (IV) oxide, mineral salts and water have to be taken into the chloroplasts.
- For respiration (energy production) to take place, food substrate such as glucose and oxygen have to be taken into the mitochondrion. Energy, carbon (IV) oxide, water and alcohol (in plants) are some of the end products of respiration.
- Some of the end products of the physiological processes such as carbon (IV) oxide can be harmful when allowed to accumulate in the cells. They, thus, have to be eliminated from the cells.
- This implies that there is a constant flow of materials in and out of the cells and the cell organelles where these physiological processes are taking place. There is a constant movement of materials across the cell membrane in the cells.
- This chapter discusses the properties of the cell membrane and the processes through which materials move in and out of the cells.

Structure of the Membrane
- A membrane is a surface structure that encloses the cell and cell organelles.
- The membranes include the cell membrane, tonoplasts, nuclei membrane, mitochondrial membrane and chloroplast membrane.
- The membranes have a common basic structure which regulates the movement of materials in and out of the cells.
- The cell membrane is made up of a phospholipid layer sandwiched by two protein layer (it is a lipoprotein layer) the overall thickness of the cell membrane is about 7.5 nm thick.
- The membrane is perforated by small pores that allow the passage of substances in and out of the cells.


Properties of the Cell Membrane
- The cell membrane is semi permeable - The pores that occur on the cell membrane allows the passage of the small size molecules but does not allow the passage of the large sized molecules. Such a membrane is said to be selectively permeable or semi-permeable. In particular, when a cell is surrounded by a dilute sugar solution, the small sized water molecules will enter the cell but the larger sugar molecules will not pass through the cell membrane. In contrast, the cell wall is permeable as it allows both sugar and water molecules to pass through it; it has larger pores. This property of selectively permeability enables the cell membrane to select what enters and leaves the cell.
- The cell membrane is sensitive to changes in temperature and pH - Cell membranes are made up of protein. Proteins are adversely affected by extreme changes in temperature and pH. Changes in temperature and pH will alter the structure of the cell membrane thereby hindering the normal functioning of the cell membrane. High temperature denatures (destroys) the proteins thereby impairing the functions of the cell membrane.
- The cell membrane possesses electric charges - The cell membrane has both positive and negative charges. These charges affect the manner in which substances move in and out of the ells. The charges also enable the cell to detect changes in the environment.

Physiological Processes of the Cell Membrane
- In this section, we discuss the various physiological processes through which materials move in and out of the cells across the cell membrane.
- Materials move in and out of the cells through three main physiological processes:
- Diffusion
- Osmosis
- Active transport

Diffusion
- From kinetic theory, matter is made up of particles that are in continuous random motion. In solids, the particles are at fixed positions and can only vibrate at these fixed positions.
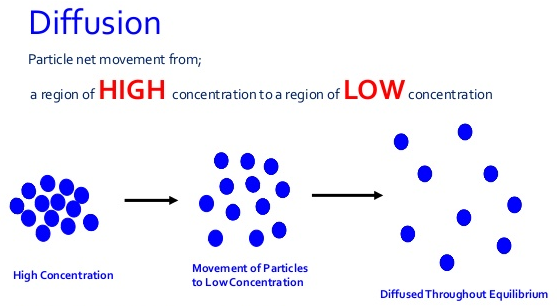
- In liquids and gases, the particles are loosely held and are free to move from one region to another randomly. This movement of gas or liquid particles is observed to be from regions of high concentration to a region of low concentration. The process by which particles move from a region of high concentration to a region of low concentration is known as diffusion.
- In particular, the scent of a flower or perfume experienced by an individual is as a result of the flower scent particles or perfume particles move from a region of high concentration.
- Diffusion occurs until the regions have an even concentration of the liquid or gas particles.
- The difference in concentration of particles between the region of high concentration and region of low concentration is known as the diffusion gradient/concentration gradient.
Demonstration of the Process of Diffusion using Potassium Manganate (VII)
Requirements:
potassium manganate (VII) crystals, glass tubing, 100 cm3 beaker and water.
Procedure
- Hold the glass tubing vertically in a beaker so that one end of the tubing rests on the bottom of the beaker.
- Cautiously and quickly drop a crystal of potassium manganate (VII) through the upper opening of the glass tubing.
- Close the upper hand of the glass tubing with the thumb.
- Half fill the beaker with water.
- Carefully withdraw vertically the glass tubing so that the crystal is left undisturbed at the bottom of the beaker.
- Record your observations for the first 15 minutes.
- Explain your observations.
Expected observations
- After some time, the purple colour of the potassium manganate (VII) spread throughout the water and eventually all the water turned purple.
Explanation
- The crystals of potassium manganate (VII) are highly concentrated with the potassium manganate (VII) particles. The potassium manganate (VII) particles break away from the crystals, dissolve in water and then diffuse through the water until they are evenlydistributed.

The Role of Diffusion in Living Organisms
-
In Plants
- Diffusion plays an important role in plants in that: It helps in absorption of mineral salts from the soil to the plant. Most salts dissolve in soil water. For those salts whose concentration in soil water is higher that their concentration in the cell sap of root hair cells, they move into the root hair cells through diffusion. Plants require mineral salts for numerous life processes.
- Diffusion plays a role in gaseous exchange in plants. The respiratory gases (oxygen and carbon (IV) oxide) diffuse across the stomata and lenticels of plants.
- Diffusion also contributes to the transportation of manufactured food materials from the leaves to other parts of the plant.
-
In Animals
- In animals diffusion plays the following important roles: It helps in the absorption of digested food materials in the alimentary canal. End products of digestion such as amino acids and glucose diffuse across the wall of the ileum into the blood for transport to other parts of the animal body.
- Diffusion also plays a significant role in gaseous exchange in animals. In animals, gaseous exchange occurs at certain structures known as respiratory surfaces. These include the skin, gills, lungs, tracheal system and the cell membrane (in unicellular organisms). Gaseous exchange at these surfaces occurs through the process of diffusion.
- Diffusion is important in excretion of nitrogenous wastes especially in unicellular animals.
Factors Affecting the Rate of Diffusion
-
Diffusion gradient
- A greater diffusion gradient between two points increases the rate of diffusion. Increasing the concentration of diffusing molecules also increases diffusion gradient with corresponding regions hence increases the rate of diffusion.
-
Surface area to volume ratio
- Rate of diffusion directly depends on the surface area to volume ratio. The greater the surface area to volume ratio, the greater the rate of diffusion will be. Conversely, low surface area to volume ratio results in a low diffusion rate.
- This implies that diffusion rate is greater in small organisms than the large organisms. This is because the small organisms have a large surface area to volume ratio. As a result, most of their body parts are closer to the external surrounding leading to faster diffusion.
- Small organisms can, therefore, depend on diffusion alone as a means of transporting foods, respiratory gases and waste products.
- To large organisms, diffusion alone is inadequate as a means of transport of foods and excretion. They have an additional transport system.
- Organisms always lose heat to the surrounding through diffusion. This implies that small animals lose a lot of heat to the surrounding compared to the large animals.
-
Thickness of membranes and tissues
- The thicker the membrane or tissue, the lower the rate of diffusion. This is because the distance covered by the diffusing molecules is greater through the thicker membranes.
- The rate of diffusion is higher in thinner membranes.
-
Size of molecules
- Small and light molecules diffuse much faster than the heavy and large sized particles.
-
Temperature
- An increase in temperature increases the energy content of the diffusing particles; thereby causing them to move faster, this implies that the rate of diffusion increases with increase in temperature.

Osmosis
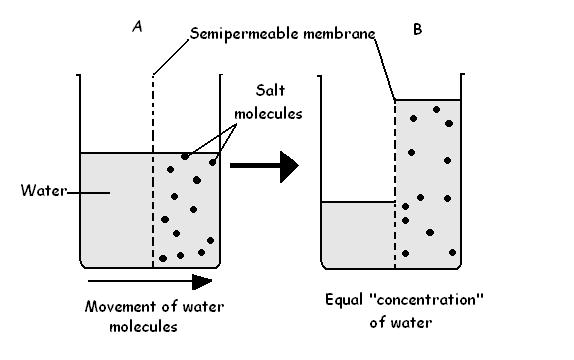
- Osmosis is a process by which solvent molecules move from a region of high concentration (dilute solution) to a region of low concentration (concentrated solution) through a semi permeable membrane.
- Osmosis can be described as a special type of diffusion since it involves movement of solvent (water) particles from a region of high concentration to a region of low concentration.
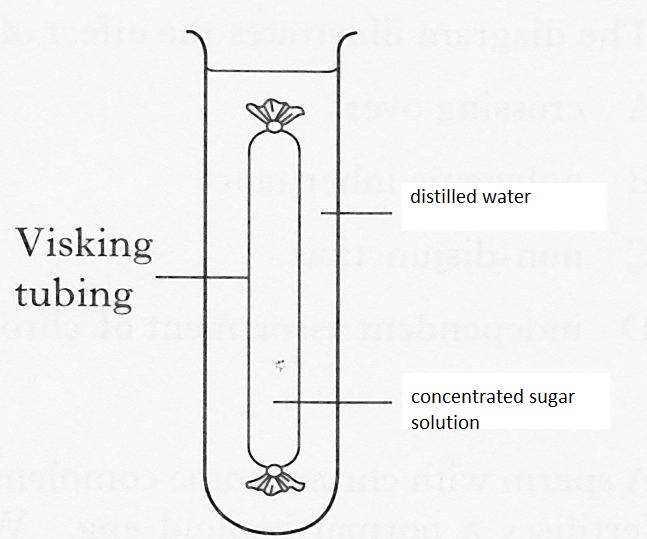
Demonstration of Osmosis Using a Visking Tubing
Requirements
500cm3 beaker, visking tubing, a piece of thread, glass rod, concentrated sugar solution, 500 cm3 distilled water.
Procedure
- Into the beaker, put 350 cm3 of the distilled water.
- Dip the visking tubing in water to moisten it. Open the visking tubing and tie one end with the thread provided.
- Half fill the visking tubing with the sugar solution provided and then tie the open end of the tubing. Ensure no sugar solution spills out of the tubing.
- Immerse the visking tubing into the distilled water in the beaker and suspend it using the glass rod provided.
- Leave the set up for about 30 minutes.
- Record your observations.
- Explain the observations made.
Observations
- The visking tubing became swollen indicating that its cell contents increased. The amount of water in the beaker decreased. This implies that water moved from the beaker into the visking tubing.
Explanation
- The visking tubing contains both sugar and water molecules. The beaker contains a higher concentration of water molecules than the visking tubing. The water molecules diffused from the beaker (where they are highly concentrated) into the visking tubing (where they are lowly concentrated). Even though there is a higher concentration of sugar molecules in the visking tubing, they were not able to diffuse out of the visking tubing due to their large molecular sizes. The visking tubing is semi permeable.
- Other than visking tubing, dialysis tubing or cellophane are also other semi permeable membranes that can be used in this experiment.
Explanation of Osmosis, Osmotic Pressure & Osmotic Potential
Explanation of Osmosis
- When two separate solutions are separated by a semi permeable membrane, there will be movement of water molecules from their region of high concentration (dilute solution) to a region of low concentration (the highly concentrated solution) across the semi permeable membrane. The semi permeable membrane does not allow movement of solute particles across it.
- The movement of the water molecules continues until the separate solutions have the same concentrations.
- Solutions with the same concentrations are referred to as isotonic solutions. The solutions are said to be isotonic to each other.
- A lowly concentrated solution (dilute solution) is referred to as a hypotonic solution. A hypotonic solution has less of the solute molecules but more of the solvent molecules.
- A highly concentrated solution with more of the solute particles but less of the solvent particles is referred to as a hypertonic solution.
- When isotonic solutions are separated with a semi permeable membrane, there will be no net movement of solvent molecules to any of the solutions since they have the same concentration of solvent molecules.
Osmotic Pressure
- When a concentrated solution is separated from distilled water by a semi permeable membrane, the concentrated solution will develop a force with which it draws water through the semi permeable membrane from the distilled water.
- Osmotic pressure refers to the force with which a concentrated solution draws water to itself.
- An osmometer is an instrument used to measure the osmotic pressure.
Osmotic Potential
- This is a measure of the pressure a solution would develop to withdraw water molecules from pure water when separated by a semi permeable membrane.
Water Relations in Animals
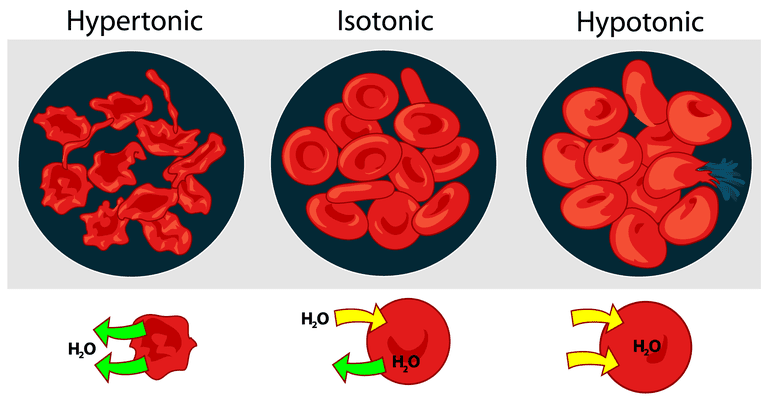
- As discussed earlier, the cell membrane is semi permeable. Let us discuss what would happen if an animal cell say red blood cell is placed in solutions of varying concentrations
-
Red blood cell in hypotonic solution e.g. distilled water
- Distilled water has a higher concentration of water molecules compared to the red blood cell cytoplasm. When a red blood cell is placed in a hypotonic solution, water will move into the cell through osmosis. The cell will swell and burst. Swelling of red blood cell when placed in a hypotonic solution is referred to as haemolysis. The cell is said to be haemolysed.
-
Red blood cell in hypertonic solution
- A hypertonic solution has a low concentration of water molecules compared to the red blood cell cytoplasm. Water will, therefore, be drawn out of the cell into the hypertonic solution. The cell will shrink and become small. The cell is said to be crenated. The process by which animal cells shrink and become smaller when placed in hypertonic solutions is referred to as crenation.
-
Red blood cell in isotonic solution
- When placed in an isotonic solution, the cell remains unchanged. This is because there will be no net inflow or outflow of water between the cell and the solution.
-
Note:
- When the cell becomes haemolysed or crenated, its functioning is impaired. This implies that the body fluids and blood plasma surrounding the cells must be kept at the same concentration as the animal cells. This will prevent bursting or shrinking of the cells that would otherwise impair their physiology.
- The body has a mechanism through which these concentrations are maintained at a nearly same concentration.
Water Relations in Plants
- Water relations in plant cells differ with that in animal cells.
- A plant cell has both a cellulose cell wall and cell membrane. The centre of the cell contains vacuole with sap. The sap is a solution of salts and sugars and is bound by a membrane, the tonoplast.
- The cell membrane and tonoplast are semi permeable while the cellulose cell wall is fully permeable.
-
Plant cell in hypotonic solution e.g. distilled water
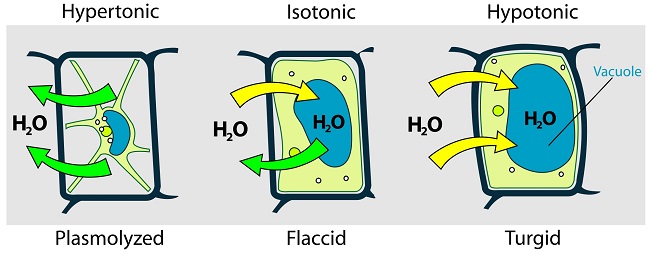
- If a plant cell is placed in water or hypotonic solution, the cell will draw water from the hypotonic solution through osmosis causing the cell to distend.
- The cellulose cell wall is rigid and does not allow plant cells to burst as in the case of animal cells.
- As the cell gains more water, the vacuole enlarges and exerts an outward an outward pressure on the cell wall called turgor pressure.
- The turgor pressure increases as more water is taken into the vacuole causing the cell to stretch until the cell cannot stretch any more. The cell becomes firm and is said to be turgid.
- Turgor pressure is the outward pressure that the cell cytoplasm exerts on the cell wall as it gains more water through osmosis.
- When the cell wall is being stretched towards the outside, it will develop a resistant pressure to stretching that is equal and opposite to turgor pressure called wall pressure.
-
A plant cell in a hypertonic solution
- When placed in a hypertonic solution, the plant cell will lose water to the solution through osmosis. As the water moves out of the cell, the cell starts to shrink, becomes less rigid or flabby and is said to be flaccid.
- If the cell loses more water, its contents reduce in size and the plasma membrane pulls away from the cell wall towards the centre. The process through which plant cells lose water, shrink and become flaccid is called plasmolysis.
- Plasmolysis can be reversed when a flaccid cell is placed in distilled water in a process called deplasmolysis.
-
Wilting
- Plants always lose water to the atmosphere through transpiration and evaporation. Simultaneously, the plant cells lose water and draw more from the soil.
- Wilting is a phenomenon that occurs when plant cells lose more water than they draw from the soil making the plant cells to lose their turgor pressure and droop.
- At night, plants always recover from wilting since stomata are closed and water loss through evapotranspiration is significantly reduced.
- Where water supply from the soil is inadequate, the plants may fail to recover from wilting and instead undergo permanent wilting.
-
Role of Osmosis in Organisms
- Absorption of water from the soil - The root hair cell of plants absorbs water from the soil through osmosis. Osmosis also helps in distribution and movement of water from the roots to other parts of the plant.
- Osmosis plays an important role in support in herbaceous plants and young seedlings. When the cells of these plants take in water through osmosis, the cells become firm or rigid and thus gain support.
- Osmosis plays a role in opening and closing of stomata in plants - The guard cells surrounding the stomata synthesize glucose through photosynthesis in the presence of light. As glucose accumulates in the guard cells, the osmotic pressure of the guard cells increase making them to draw water from adjacent cells through osmosis. When the guard cells become turgid, they bulge outwards leading to opening of the stomata. Opening of the stomata is crucial as it allows for gaseous exchange in plants. At night, there is no glucose synthesis. The glucose available in the guard cells is respired on leading to reduction of glucose and consequently reduction in osmotic pressure. The guard cells lose turgidity and close the stomata.
- Osmosis also plays a role in feeding in insectivorous plants - These plants live on nitrogen deficient soils and trap insects from whence they obtain the nutrients. These plants possess special structures that suddenly change their turgor pressure when disturbed. The change in turgor pressure enables the special structures to rapidly close thereby trapping the insects.
- Osmosis also plays a role in osmoregulation in animals.
- In kidney tubules of animals, water is withdrawn from the tubules into the body cells through osmosis through the tubular walls. This enables animals to maintain the osmotic pressure of the body fluids.
Factors Affecting the Rate of Osmosis
- Concentration of solutions and concentration gradient. Osmosis is greater when the separated solutions have a greater difference in osmotic pressure. In summary, the greater the concentration gradient, the greater the rate of osmosis and vice versa.
- Temperature - An increase in temperature would increase the rate of osmosis as it increases the energy content of the molecules.
Active Transport
- Active transport refers to the process through which substances are moved across the cell membrane and against a concentration gradient.
- Diffusion and osmosis alone do not account for movement of substances in and out of the cells. In particular, there are some mineral salts that occur at low concentrations in the soil water than in the cell sap. Some of these mineral salts cannot be absorbed by the plants through diffusion. A mechanism that would move them into the cells against the concentration gradient will be useful.
- Active transport requires energy. This is unlike diffusion and osmosis that only depend on concentration gradient for them to take place.
- It is postulated that there are protein carrier molecules on the cell membrane that aid in the moving these substances across the membrane. These carrier molecules combine with the substances being transported across the membrane and then move them from one side of the membrane to the other side.
- Cellular intake of solutes is largely through active transport.
Role of Active Transport in Living Organisms
- It helps in re-absorption of sugars and some salts by the kidney to the bloodstream.
- It helps in absorption of some mineral salts from the soil by roots.
- Absorption of digested food from alimentary canal of animals into the bloodstream.
- It leads to accumulation of substances into the body to offset osmotic imbalance in arid and saline environments
- It plays a role in excretion of waste products from body cells.
Factors Affecting the Rate of Active Transport
Most factors that affect active transport are those factors that would affect the energy production process in living cells.
These include:
-
Oxygen concentration
Oxygen is required in respiration process that yields energy for active transport. Under low oxygen concentration, the rate of respiration will be low hence there will be production of little energy leading to low rate of active transport. Increase in oxygen concentration translates into a higher energy production leading to high rate of active transport. -
Change in pH
Change in pH affects the respiratory process which is enzyme controlled. Respiratory enzymes require optimum pH for their efficient activity. Extreme pH conditions will lower the rate of active transport since the enzymes controlling respiration will be denatured. -
Glucose concentration
Glucose is the chief respiratory substrate. At low glucose concentration, there will be less production of energy leading to decreased rate of active transport. Rate of active transport increases with increase in glucose concentration due to increase in the rate of energy production. -
Temperature
Temperature affects the enzyme controlled respiration process. At low temperatures, the enzymes are inactive hence the rate of respiration will be low resulting into low rate of active transport since there will be less production of energy. An increase in temperature increases the rate of respiration since the enzymes become more activated. At temperatures beyond 40 degrees celcius, the enzymes become denatured, respiration stops and so does active transport. -
Presence of metabolic inhibitors e.g. cyanide.
These are substances which act as metabolic poisons. They stop the rate of respiration leading to production of no energy. Active transport is, thus, stopped.
Download Cell Physiology - Form 1 Biology Notes.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students