INSTRUCTIONS TO CANDIDATES
- Write your name, School and Index Number in the spaces provided above.
- Sign and write date of examination in the spaces provided above.
- Answer ALL questions in the spaces provided.
- Mathematical tables and silent non-programmable electronic calculators may be used.
- All working MUST be clearly shown where necessary.
- Candidates should answer the questions in English
- Candidates should check the question paper to ascertain that all pages are printed as indicated and that no questions are missing
- This paper contains 9 printed pages
-
- The information below relates to element N, P,Q, Rand S . Study it and answer the questions that follow. The letters are not the actual symbols for the elements.
Element Atomic radius(mm) Ionic radius(mm) Formula of oxide Melting point of oxide N 0.364 0.364 N2O -119 P 0.830 0.711 PO2 837 Q 0.592 0.485 Q2O3 1466 R 0.381 0.446 R2O5 242 S 0.762 0.676 SO 1054 - Name the elements that are metal. Give a reason. (2mks)
- Compare the melting points of the oxides of S and R in terms of structure and bonding. (2mks)
- Name the pair of elements that would react most vigorously with each other?
Explain (2mks)
- The table bedlow has information about chlorides of elements in period 3 of the periodic table:
Sulphur to sulphur
What are the possible PH values of the solutions formed when the following chlorides are dissolved in water? ExplainChloride NaCl MgCI2 AICI3 SiCI4 PCI5 Melting point (ºC) 801 712 Sublimes at 183 -70 -80
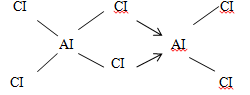
MgCI2 (1mk) - The molecular formula of Aluminum chloride is AI2CI6. Draw the structural (not dot and cross diagram) of Aluminum chloride indicating clearly the different types of bonds present. (2mks)
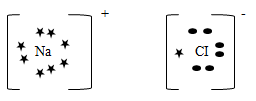
- Using dot (•) and cross (×), draw a diagram to show bonding in sodium chloride. (Na=11, CI=17) (2mks)
- The information below relates to element N, P,Q, Rand S . Study it and answer the questions that follow. The letters are not the actual symbols for the elements.
-
- What is the molar enthalpy of neutralization? (1mk)
- In order to determine the molar heat of neutralization of sodium hydroxide, 100cm3 of 1M sodium hydroxide and 1M of hydrochloric acid both at the initial temperature were mixed and stirred continuously using a thermometer. The temperature of the resulting solution was recorded after every 30seconds until the highest temperature was attained. Thereafter the temperature of the solution was recorded for a further two minutes.
- Why was it necessary to stir the mixture of the two solutions? (1mk)
- Write an ionic equation for the reaction that took place. (1mk)
- The sketch below was obtained when temperature of the mixture was plotted against time. Study it and answer the questions that follow.
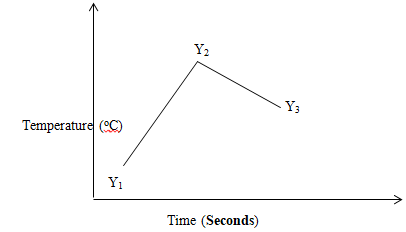
Explain the temperature changes between points
Y1 and Y2 (1mk)
Y2 and Y3 (1mk) - If the initial temperature for both solution was 25ºC and the highest temperature was 31.4ºC for the mixture. Calculate;
Heat change for the reaction (Specific heat capacity of solution=42Hg-1K-1, Density of the solution =1gcm-3) (2mks)
Molar heat of neutralization of sodium hydroxide. (2mks) - Explain how the molar heat of neutralization obtained in this experiment would compare with one that would be obtained using 1.0M ethanoic acid and 100cm3 of 1M sodium hydroxide solution. (2mks)
Draw an Energy level diagram for the reaction represented by reaction between hydrochloric acid and sodium hydroxide solution. (3mks)
-
- Give the name of one reagent which when reacted with concentrated hydrochloric acid produces chlorine gas. (1mk)
- The set up below was used to prepare iron (III) chloride using the apparatus shown in the diagram below.
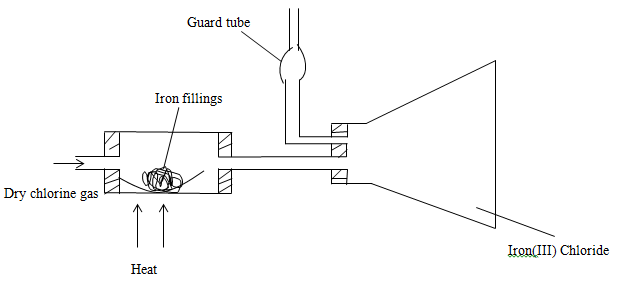
- State one precaution that should be taken in carrying out the above experiment. (1mk)
- Explain why
Calcium oxide would be preferred to calcium chloride in the guard tube. (2mks)
It is necessary to pass chlorine gas through the apparatus before heating begins. (2mks) - Write a chemical equation for the reaction that took place in the guard tube.(1mk)
- What property of Iron (III) chloride makes it possible to be collected as shown in the diagram? (1mk)
- During the reaction in the combustion tube, the total mass of iron (III) chloride formed was found to be 1.5g. Calculate the volume of chlorine gas that reacted with iron. (Fe=56.0, CI=35.5 and molar gas volume at 298k is 24,000cm3) (3mks)
- Draw and name the structure of the compound formed when excess chlorine gas is reacted with ethane gas. (2mks)
- State one use of chlorine gas. (1mk)
-
- Give the systematic names for the following compounds
- HCOOCH2CH3 (1mk)
- CH3CH2CH2CHCH2 (1mk)
- CH C CH2 CH3(1mk)
- Study the flow chart below and use it to answer the questions that follow
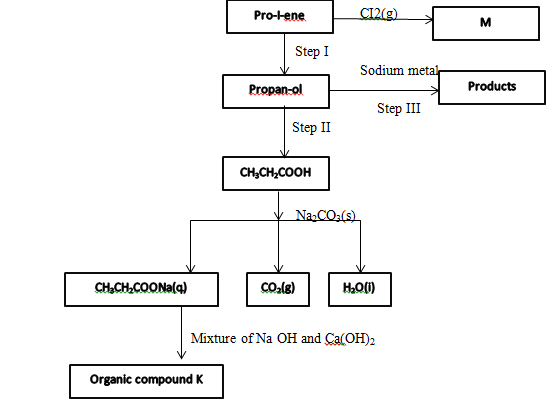
- Identify the organic compound K (1mk)
- Write the formula of M (1mk)
- Give one reagent that can be used in
Step I (1mk)
Step II (1mk) - Write the equations for the reaction in step II (1mk)
- The structure below represents a type of cleansing agent.
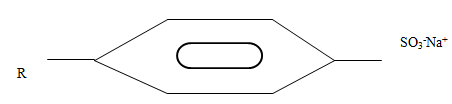
Describe how the cleansing agent removes grease from a piece of cloth. (3mks)
- Give the systematic names for the following compounds
-
- The set up below was used to collect gas F, produces by the reaction between water and calcium metal
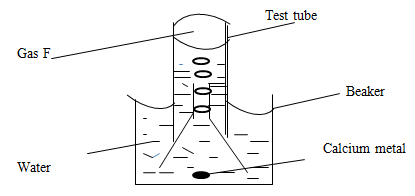
- Name gas F (1mk)
- At the end of the experiment, the solution in the beaker was found to be a week base. Explain why the solution is a weak base. (2mks)
- Give one laboratory use of the solution formed in the beaker (1mk)
- When excess calcium metal was added to 50cm3 of 2 M aqueous copper (II)nitrate in a beaker, a brown solid and bubbles of gas were observed.
- Write two equations for the reactions which occurred in the beaker. (2mks)
- Explain why it is not advisable to use sodium metal for this reaction. (2mks)
- Calculate the mass of calcium metal reacted with copper(II)nitrate solution (Relative atomic mass of Ca=40 (2mks0
- The set up below was used to collect gas F, produces by the reaction between water and calcium metal
-
- Write the formula of the complex Ion formed in each of the reactions below.
- Lead metal dissolves in hot alkaline solution. (1mk)
- Zinc hydroxide dissolves ammonia solution. (1mk0
- Give the name of each of the processes described below which takes place when the salts are exposed to air for some time.
- Anhydrous copper (II) sulphate becomes wet. (1mk)
- Iron (III) chloride forms an aqueous solution. (1mk)
- Fresh crystals of sodium carbonate decahydrate become covered with a powder of solution of carbonate monohydrate. (1mkl)
- A certain hydrate salt has the following composition by mass. Iron 20.2%, sulphur 11.5%, water 45.5% and the rest oxygen. Its relative formula mass is 278.
- Determine the formula of the hydrated salt (Fe=56,S=52, O=16, H=1) (3mks)
- 6.95g of the hydrated salt were dissolved in distilled water and the total volume made to 250cm3 of solution. Calculate the concentration of the salt solution. (2mks)
- Write the formula of the complex Ion formed in each of the reactions below.
- The table below shows solubility of potassium nitrate and lead nitrate
Temperature ºC 0 20 40 60 80 100 Solubility of KNO3 in 100g of H2O 12.5 32.5 62.5 110.0 137.5 Solubility of Pb(NO3)in 100g of H2O 37.5 52.5 69.0 87.5 110.0 131.0 - Draw the solubility curves for both salts on the same axis. (Temperature on the x-axix) (3mks)
- A solution of lead nitrate contains 90g of the salt dissolved in 100g of water at 100ºC. This solution is allowed to cool to 25ºC
At what temperature will crystals first appear? (1mk)
What mass of crystals will be present at 25ºC (1mk) - Which of the two salts is more soluble at 30ºC (1mk)
- Determine the concentration of lead nitrate in moles per litre when the solubility of the two salts are the same. (Pb=207.0, O=16.0,
- Draw the solubility curves for both salts on the same axis. (Temperature on the x-axix) (3mks)

MARKING SCHEME
-
- The information below relates to element N, P,Q, Rand S . Study it and answer the questions that follow. The letters are not the actual symbols for the elements.
Element Atomic radius(mm) Ionic radius(mm) Formula of oxide Melting point of oxide N 0.364 0.364 N2O -119 P 0.830 0.711 PO2 837 Q 0.592 0.485 Q2O3 1466 R 0.381 0.446 R2O5 242 S 0.762 0.676 SO 1054 - Name the elements that are metal. Give a reason. (2mks)
PQRS; Ionic radius is smaller than atomic radius - Compare the melting points of the oxides of S and R in terms of structure and bonding. (2mks)
S has higher melting point than R; SO has a simple molecular structure(or in terms of bonds) - Name the pair of elements that would react most vigorously with each other?
Explain (2mks)- P and N; P is a metal with the smallest atomic radius ;
- N is a non-metal with the smallest atomic radius
- Name the elements that are metal. Give a reason. (2mks)
- The table below has information about chlorides of elements in period 3 of the periodic table:
Sulphur to sulphur
What are the possible PH values of the solutions formed when the following chlorides are dissolved in water? ExplainChloride NaCl MgCI2 AICI3 SiCI4 PCI5 Melting point (ºC) 801 712 Sublimes at 183 -70 -80
MgCI2 (1mk)- PH 7.0; a chloride of group II element.
AICI3 (1mk) - PH 3.0; It hydrolyses in water to form HCI acid.
- PH 7.0; a chloride of group II element.
- The molecular formula of Aluminum chloride is AI2CI6. Draw the structural (not dot and cross diagram) of Aluminum chloride indicating clearly the different types of bonds present. (2mks)
- Using dot (•) and cross (×), draw a diagram to show bonding in sodium chloride. (Na=11, CI=17) (2mks)
- The information below relates to element N, P,Q, Rand S . Study it and answer the questions that follow. The letters are not the actual symbols for the elements.
-
- What is the molar enthalpy of neutralization? (1mk)
The enthalpy change/ heat change when one mole of H+ ions react with one mole of OH- ions to form one mole of water; OWTTE - In order to determine the molar heat of neutralization of sodium hydroxide, 100cm3 of 1M sodium hydroxide and 1M of hydrochloric acid both at the initial temperature were mixed and stirred continuously using a thermometer. The temperature of the resulting solution was recorded after every 30seconds until the highest temperature was attained. Thereafter the temperature of the solution was recorded for a further two minutes.
- Why was it necessary to stir the mixture of the two solutions? (1mk)
To obtain a uniform mixture of reagents; uniform distribution of heat; - Write an ionic equation for the reaction that took place. (1mk)
H+(aq) + OH-(aq) H2O(l)
H3O+(aq) + OH-(aq) 2H2O(l)
Penalise ½mk for missing SS - The sketch below was obtained when temperature of the mixture was plotted against time. Study it and answer the questions that follow.
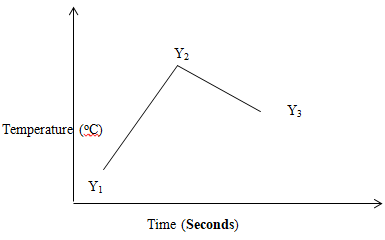
Explain the temperature changes between points
Y1 and Y2 (1mk)
Reaction is taking place ; producing heat;/Reaction is exothermic;
Y2 and Y3 (1mk)
Reaction has come to an end; the mixture is cooling; - If the initial temperature for both solution was 25ºC and the highest temperature was 31.4ºC for the mixture. Calculate;
Heat change for the reaction (Specific heat capacity of solution=42Hg-1K-1, Density of the solution =1gcm-3) (2mks)
DT=31.4-25=6.4oC
Heat change =200×6.4×4.2
=5376J//5.376KJ
Molar heat of neutralization of sodium hydroxide. (2mks)
Moles of NaOH = 100 ×1 =0.1Moles
1000
0.1 moles=5376
1mole=5376 × 1
0.1 1000
=53.76KJ/Mole - Explain how the molar heat of neutralization obtained in this experiment would compare with one that would be obtained using 1.0M ethanoic acid and 100cm3 of 1M sodium hydroxide solution. (2mks)
Lower; Ethanoic acid is partially ionized/dissociated;//week acid// fewer H+ions thus some energy is used to change the unionised molecules into ions first;
Draw an Energy level diagram for the reaction represented by reaction between hydrochloric acid and sodium hydroxide solution. (3mks)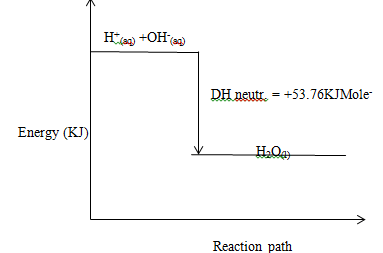
- Why was it necessary to stir the mixture of the two solutions? (1mk)
- What is the molar enthalpy of neutralization? (1mk)
-
- Give the name of one reagent which when reacted with concentrated hydrochloric acid produces chlorine gas. (1mk)
Potassium manganite(vii)//Manganese(iv)oxide//Lead (iv)oxide//Calcium hypochlorite
ALC KMnO4//MnO2/PbO2/CaOCI2 - The set up below was used to prepare iron (III) chloride using the apparatus shown in the diagram below.
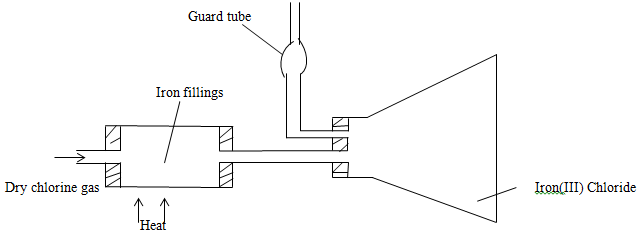
- State one precaution that should be taken in carrying out the above experiment. (1mk)
The experiment should be carried out in a fume cupboard; CI2 should not be allowed to escape into the environment since it’s poisonous/toxic. - Explain why
Calcium oxide would be preferred to calcium chloride in the guard tube. (2mks)
CaO absorbs CI2 and moisture; while CaCI2 only absorb moisture;
CaO absorbs only CI2 award 1mark
CaCI2 absorbs only moisture
It is necessary to pass chlorine gas through the apparatus before heating begins. (2mks)
To remove all air/oxygen; which would react with iron //form Iron (III) oxide; instead of Iron (III) Chloride. - Write a chemical equation for the reaction that took place in the guard tube.(1mk)
CaO(s) + H2O(l) Ca(OH)2(s)/(aq)
//CaO(s) + CI2(g) CaOCI2(s)
//Ca(OH)2(s) +CI2(g) CaOCI2. H2O(s) - What property of Iron (III) chloride makes it possible to be collected as shown in the diagram? (1mk)
It sublimes - During the reaction in the combustion tube, the total mass of iron (III) chloride formed was found to be 1.5g. Calculate the volume of chlorine gas that reacted with iron. (Fe=56.0, CI=35.5 and molar gas volume at 298k is 24,000cm3) (3mks)
2Fe(s) +3CI2(g) 2FeCI3(s) Ignore ss
RFM of FeCI3=162.5
Moles of FeCI3 = 1.5 =0.0092
16.3
Moles of CI2 =3/2 × 0.0092 =0.0138
Volume of CI2 =0.0138 × 24000
=332.31cm3
// 2Fe(s) +3CI2 2FeCI3(s)
3 × 24000=162.5 × 2
3×24000×15 =332.31cm3
162.5×2
- State one precaution that should be taken in carrying out the above experiment. (1mk)
- Draw and name the structure of the compound formed when excess chlorine gas is reacted with ethane gas. (2mks)
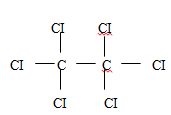
- State one use of chlorine gas. (1mk)
- Manufacture of hydrochloric acid
- Treatment of Cvuter
- Manufacture of PVC Any other possible use
- Give the name of one reagent which when reacted with concentrated hydrochloric acid produces chlorine gas. (1mk)
-
- Give the systematic names for the following compounds
HCOOCH2CH3 (1mk)- Propanoic acid/Propan –l-IOC acid
CH3CH2CH2CHCH2 (1mk) - Pent -l-eneRj. Structural formular/ 1-pentene
CH C CH2 CH3 (1mk) - But-l-yne RjS.F/l-Butyne
- Propanoic acid/Propan –l-IOC acid
- Study the flow chart below and use it to answer the questions that follow
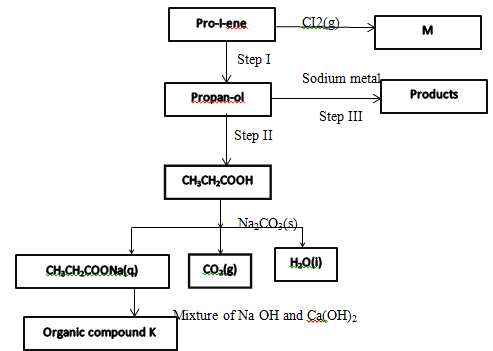
- Identify the organic compound K (1mk)
Ethane - Write the formula of M (1mk)
C3H6CI2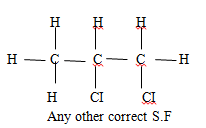
- Give one reagent that can be used in
Step I (1mk)- Water/steam/conc. Sulphuric (vi) acid; Rej. Dillute Sulphuri (vi)acid
- Sulphuric acid award ½ mark only
Step II (1mk) - Acidified Potassium manganite (vii)/Acidified potassium chromate(vii)
H+(aq)/KMnO4(aq) H+(aq) /K2CrO7(aq)
- Write the equations for the reaction in step II (1mk)
2CH3CH2CH2OH +2Na 2CH3CH2CH2ONa +H2
Ignore SS unless wrongly committed
- Identify the organic compound K (1mk)
- The structure below represents a type of cleansing agent.

Describe how the cleansing agent removes grease from a piece of cloth. (3mks)
Cleansing agent has a polar end / hydrophilic; and non-polar/hydrophobic end; polar end/ hydrophilic end; is attracted to water while non-polar/hydrophobic end; to grease; Results in formation of micelles//lower surface tension of water//emulsification of grease;
- Give the systematic names for the following compounds
-
- The set up below was used to collect gas F, produces by the reaction between water and calcium metal
- Name gas F (1mk)
Hydrogen; - At the end of the experiment, the solution in the beaker was found to be a week base. Explain why the solution is a weak base. (2mks)
Ca(OH)2 formed is slightly soluble in water hence only a few OH- ions are produced in solution - Give one laboratory use of the solution formed in the beaker (1mk)
Used for testing the presence of CO2 gas.
- Name gas F (1mk)
- When excess calcium metal was added to 50cm3 of 2 M aqueous copper (II)nitrate in a beaker, a brown solid and bubbles of gas were observed.
- Write two equations for the reactions which occurred in the beaker. (2mks)
Ca(s) + 2H2O(l) → Ca(OH)2(aq)+H2(g)
Ca(s) + Cu(NO3)2(l) → Ca(NO3)2(aq) +Cu(s)
// Ca(s) + Cu2+(aq) → Ca2+(aq) +Cu(s) - Explain why it is not advisable to use sodium metal for this reaction. (2mks)
- The reaction is explosive /highly enothermic;
- Advice; sodium is more reactive than calcium
- Write two equations for the reactions which occurred in the beaker. (2mks)
- Calculate the mass of calcium metal reacted with copper(II)nitrate solution (Relative atomic mass of Ca=400 (2mks)
Moles of Cu(NO3)2 =50 × 2=0.1moles
1000
Moles ratio from equation above= 1:1
Moles of Ca=0.1
Moles of Ca=0.1 × 40
= 4g
- The set up below was used to collect gas F, produces by the reaction between water and calcium metal
-
- Write the formula of the complex Ion formed in each of the reactions below.
- Lead metal dissolves in hot alkaline solution. (1mk)
[Pb(OH)4]2- - Zinc hydroxide dissolves ammonia solution. (1mk)
[Zn(NH3)4]2+
- Lead metal dissolves in hot alkaline solution. (1mk)
- Give the name of each of the processes described below which takes place when the salts are exposed to air for some time.
- Anhydrous copper (II) sulphate becomes wet. (1mk)
Hygrosiopy - Iron (III) chloride forms an aqueous solution. (1mk)
Deliquence - Fresh crystals of sodium carbonate decahydrate become covered with a powder of solution of carbonate monohydrate. (1mk)
Efflorence
- Anhydrous copper (II) sulphate becomes wet. (1mk)
- A certain hydrate salt has the following composition by mass. Iron 20.2%, sulphur 11.5%, water 45.5% and the rest oxygen. Its relative formula mass is 278.
- Determine the formula of the hydrated salt (Fe=56,S=52, O=16, H=1) (3mks)
FeSO 4.7H2OElement Fe S O H2O % Composition 20.2 11.5 22.8 45.5 R.A.M 56 32 16 18 Ratio % 20.2=0.36
56
0.36 =1
0.3611.5=0.36
32
0.36 =1
0.3622.8
16
1.43 =4
0.3645.5 =2.5
18
2.5 =7
0.36 - 6.95g of the hydrated salt were dissolved in distilled water and the total volume made to 250cm3 of solution. Calculate the concentration of the salt solution. (2mks)
6.95g in 250cm3
X=1000cm3
= 6.95 × 4000cm3 =0.1M
250cm3 × 278
- Determine the formula of the hydrated salt (Fe=56,S=52, O=16, H=1) (3mks)
- Write the formula of the complex Ion formed in each of the reactions below.
- The table below shows solubility of potassium nitrate and lead nitrate
Temperature ºC 0 20 40 60 80 100 Solubility of KNO3 in 100g of H2O 12.5 32.5 62.5 110.0 137.5 Solubility of Pb(NO3)in 100g of H2O 37.5 52.5 69.0 87.5 110.0 131.0 - Draw the solubility curves for both salts on the same axis. (Temperature on the x-axis) (3mks)
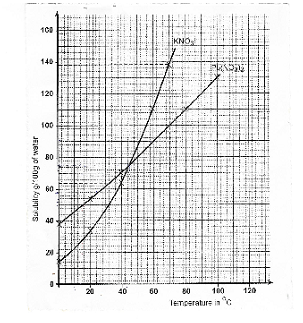
- A solution of lead nitrate contains 90g of the salt dissolved in 100g of water at 100ºC. This solution is allowed to cool to 25ºC
- At what temperature will crystals first appear? (1mk)
60ºC - What mass of crystals will be present at 25ºC (1mk)
90g-58=32g
Correct value of solubility at 25ºC
- At what temperature will crystals first appear? (1mk)
- Which of the two salts is more soluble at 30ºC (1mk)
Lead nitrate Pb(NO3)2 - Determine the concentration of lead nitrate in moles per litre when the solubility of the two salts are the same. (Pb=207.0, O=16.0, K=39.0, N=14.0) (3mks)
Solubility of Pb(NO3)2 =75g/100water
Value read from the graph
Mass of P(NO3)2 in 100cm3=75 × 100
100
Value from graph × 100
100
=75g
Molar mass of Pb(NO3)2 =331
Conc. Of Pb(NO3)2 = 75 =0.2266M // answer (i) =answer(ii) M
331 331
- Draw the solubility curves for both salts on the same axis. (Temperature on the x-axis) (3mks)
Download Chemistry Paper 2 Questions and Answers - Samia Joint Mock Examination 2021/2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

