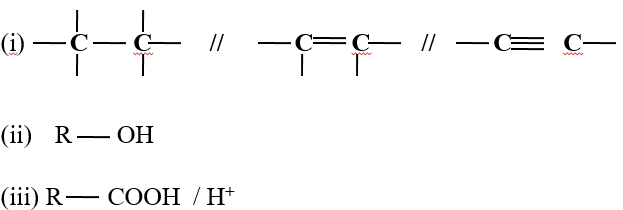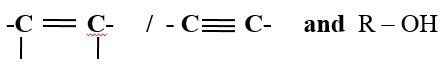Process of identifying unknown compounds
Compounds may be:
- Inorganic
- organic
Inorganic analysis
This involve mainly identification of ionic compounds containing cations and anions.
Cations present in an ionic compounds are identified by adding a precipitating reagent that forms a precipitate unique to the cation/s in the compound.
The main precipitating reagents used are:
2M NaOH and/or 2M NH3(aq)
When using 2M sodium hydroxide:
- No white precipitate formed if K + and Na + ions are present
- No white precipitate formed if NH4+ ions are present but a colourless gas with pungent smell of urine is produced which may not be recognized in a school laboratory examination setting.
- White precipitate that dissolves / soluble in excess if Zn2+ Pb2+ Al3+ ions are present.
- White precipitate that do not dissolves/insoluble in excess if Ba2+ Mg2+ Ca2+ ions are present.
- Blue precipitate that do not dissolves /insoluble in excess if Cu2+ ions are present.
- Green precipitate that do not dissolves/insoluble in excess if Fe2+ ions are present.
- Brown precipitate that do not dissolves/insoluble in excess if Fe3+ ions are present.
When using 2M aqueous ammonia
- No white precipitate is formed if K+ ,NH4+ Na+ ions are present
- White precipitate that dissolves / soluble in excess if Zn2+ ions are present.
- White precipitate that do not dissolves/insoluble in excess if Ba2+ Mg2+ Ca2+ Pb2+ Al3+ ions are present.
- Blue precipitate that dissolves /soluble in excess to form a deep/royal blue solution in excess if Cu2+ ions are present.
- Green precipitate that do not dissolves/insoluble in excess if Fe2+ ions are present.
- Brown precipitate that do not dissolves/insoluble in excess if Fe3+ ions are present.
Anions present in an ionic compounds are identified by adding a specific precipitating reagent that forms a precipitate unique to the specific anion/s in the compound.
Lead(II)nitrate(V) solution
Lead forms insoluble PbSO4 ,PbSO3 ,PbCO3, PbS, PbI2,PbCl2
PbS is a black precipitate,
PbI2 is a yellow precipitate.
All the others are white precipitates.
- If a Lead(II)nitrate(V) solution is added to a substance/ solution/ compound :
- A yellow ppt shows presence of I- ions
- A black ppt shows presence of S2- ions
- A white ppt shows presence of SO42- ,SO32- ,CO32- Cl-
- If the white precipitate is added dilute nitric(V) acid
-
It dissolves to show presence of SO32- ,CO32-
- It persist/remains to show presence of SO42-, Cl-
-
- If the white precipitate in b(i) is added acidified potassium manganate(VII)/ dichromate(VI)
-
acidified potassium manganate(VII) is decolorized /orange colour of acidified potassium dichromate(VI) turns to green to show presence of SO32-
- acidified potassium manganate(VII) is not decolorized /orange colour of acidified potassium dichromate(VI) does not turn to green/remains orange to show absence of SO32- /presence of CO32-
-
- If the white precipitate in b(ii) is boiled:
-
It dissolves to show presence of Cl-
- It persist/remains to show presence of SO42-
-
Barium(II)nitrate(V)/Barium chloride solution
Barium(II)nitrate(V)/Barium chloride solution precipitates BaSO4 ,BaSO3 , BaCO3, from SO42- ,SO32- ,CO32- ions.
Inorganic qualitative analysis require continous practice discussion
Organic analysis
This involve mainly identification of the functional group:
These functional groups can be identified by:
- burning-a substance which “catches fire” must reduce in amount. Candidates should not confuse burning with flame coloration/test
- Decolorization of bromine water/chlorine water/acidified KMnO 4 / to show presence of
- Turning orange acidified K2Cr2O7 to green to show presence as in above.
- pH 1/2/3 for strongly acidic solutions. pH 4/5/6 for weakly acidic solutions
- Turning blue litmus paper red. red litmus paper remaining red show presence of H+ ions
Download Qualitative analysis - KCSE Chemistry Paper 3 - Practicals.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students