Glossary of terms (Unique words)
- Ion
- Wavelengths of light
What is a flame test?
- Flame test is used to identify metal ions in samples. That is when a specific metal ion is put under a flame what colour does it burn with?
Why use a flame test?
- Flame tests as methods of analysing samples are faster, more accurate and more sensitive than simple chemical tests.
Though remember, using heat on sample can change a sample chemically and physically.
Why do different metal ions produce different flame colours?
The answer is in the question. Because different metals contain different ionic properties.
Flame tests rely on the unique electron configurations of each and every element in order to identify them. When in compounds as these elements often are, they exist in the form known as an ion. A metal ion exists when the atom releases its valence electrons to bond with a non-metal atom as an ionic bond. The electrons of the ion surround the nucleus in the form of orbitals. Each orbital exists at a specific energy level, where electrons tend to be near at all times.
Heating metals strongly over an open flame such as a Bunsen Burner the electrons inside of the ions become excited. The excitement stems from the added energy to the compound, and that energy being absorbed by the ion. The electrons of the ion absorb the energy and the electron will jump up an energy level. Within moments, the energy is released by the electron in order to drop back down to its ground state. The released energy is visible to the human eye in the form of unique wavelengths of light. Each metal ion releases just slightly different wavelengths of light due to each element having its own unique electron configuration. The image shows the general concept of an atom that has electrons in several different orbital locations and energy levels. The arrow in the image represents the idea of the absorption and release of energy which causes a jump and then returns to the ground state.
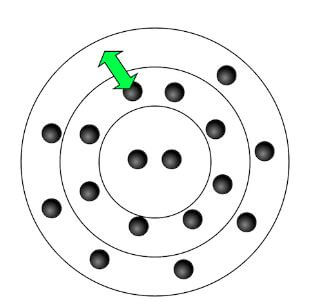
Note: Not all metal ions give flame colours.
How to carry out a flame test.
(also see the figure below)
- dip a clean wire loop into a solid sample of the compound being tested
- put the loop into the edge of the blue flame from a Bunsen burner
- observe and record the flame colour produced
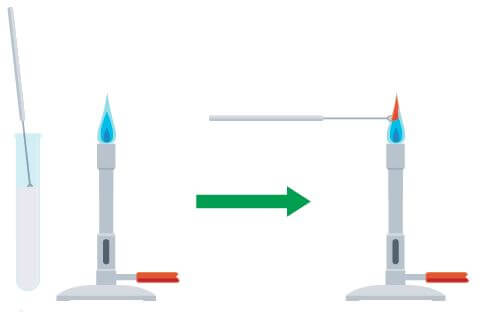
For a valid flame experiment note:
- The colour change on a clear colourless Bunsen flame is useful in identifying some cations / metals.
- A very clean metallic spatula is recommended since dirt obscures /changes the correct coloration.
Distinct flame coloration of some compounds
| Ion present | Flame test colour |
|---|---|
| Lithium, Li+ | Red |
| Sodium, Na+ | Yellow |
| Potassium, K+ | Lilac (pink) |
| Calcium, Ca2+ | Orange-red |
| Barium, Ba2+ | Green/pale-green |
| Copper, Cu2+ | Blue-green (often with white flashes) |
| Rubidium, Rb+ | Red (Reddish-violet) |
Practice Revision Questions
-
- Name the Group 1 metal whose compounds give a lilac flame colour.
- Name the two Group 1 metals whose compounds give a red flame colour.
- If you did a flame test of a Group 1 metal compound and got a red flame colour, how could you decide which metal was in the compound?
- Considering metals from other parts of the Periodic Table, which metal forms compounds which give
- a pale green flame colour;
- a blue-green flame colour?
- If you heat a compound containing Group 1 metal ions strongly in a bunsen flame, some of the ions are converted back to atoms. Heat energy from the flame promotes electrons in the atoms to empty higher energy levels. Explain how this results in a characteristic flame colour for the particular metal.
Answers
-
- potassium
- lithium and rubidium
- Compare the colour side-by-side with the flame colour produced by known lithium and rubidium compounds.
-
- barium
- copper
- The promoted electrons will lose energy again by falling back to lower energy levels. The energy released will depend on the energy gaps between the various orbitals, and this varies from element to element. Each energy jump downwards will be seen as a particular wavelength (or frequency) of light. That means that each element, with different gaps between the various energy levels, will produce a unique set of spectral lines, and so a unique flame colour
Download Flame test - KCSE Chemistry Paper 3 - Practicals.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students