The Atmosphere
The atmosphere is made up of air. Air is a mixture of colourless, odorless gases which is felt as wind (air in motion).
All living things breath in air for respiration. Plants use air for respiration and photosynthesis.
The main gases present in the atmosphere/air:
| Gas | Approximate % composition by volume |
| Nitrogen | 78.0 |
| Oxygen | 21.0 |
| Carbon(IV)oxide | 0.03 |
| Noble gases | 1.0 |
| Water vapour | Vary from region |
The following experiments below shows the presence and composition of the gases in air/atmosphere
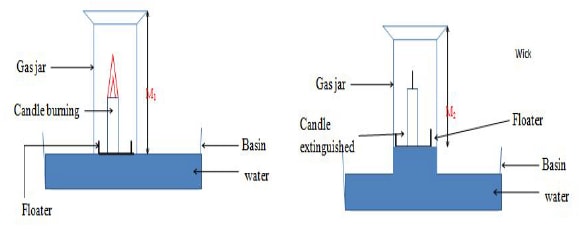
To find the composition of air supporting combustion using a candle stick
Procedure
- Measure the length of an empty gas jar M1.
- Place a candle stick on a Petri dish.
- Float it on water in basin/trough.
- Cover it with the gas jar.
- Mark the level of the water in the gas jar M2.
- Remove the gas jar.
- Light the candle sick.
- Carefully cover it with the gas jar.
- Observe for two minutes.
- Mark the new level of the water M3.
Set up of apparatus
Sample observations
Candle continues to burn then extinguished/goes off
Level of water in the gas jar rises after igniting the candle
Length of empty gas jar = M1 = 14cm
Length of gas jar without water before igniting candle = M2 = 10 cm
Length of gas jar with water before igniting candle = M1 - M2 = 14- 10 = 4 cm
Length of gas jar with water after igniting candle = M3 = 8 cm
Length of gas jar without water after igniting candle = M1 - M3 = 10 -8 = 2 cm
Explanation
Candle burns in air. In a closed system (vessel), the candle continues to burn using the part of air that support burning/combustion.
This is called the active part of air. The candle goes off/extinguished when all the active part of air is used up.
The level of the water rises to occupy the space /volume occupied by the used active part of air.
The experiment is better when very dilute sodium/potassium hydroxide is used instead of water. Dilute Potassium/ sodium hydroxide absorb Carbon (IV) oxide gas that comes out from burning/combustion of candle stick.
From the experiment above the % composition of the:
- Active part of air can be calculated:
(M2 - M3) x 100% → (10 - 8) x 100% = 20% - Inactive part of air can be calculated:
100% - 20% = 80%
M3 → 8 x 100% = 80%
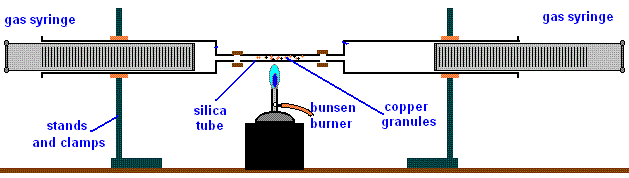
To find the composition of active part of air using heated copper turnings.
Procedure
- Clamp a completely packed/filled open ended glass tube with copper turnings.
- Seal the ends with glass/cotton wool.
- Label two graduated syringes as “A” and “B”
- Push out air from syringe “A”.
- Pull in air into syringe “B”.
- Attach both syringe “A” and “B” on opposite ends of the glass tube.
- Determine and record the volume of air in syringe “B” V1.
- Heat the glass tube strongly for about three minutes.
- Push all the air slowly from syringe “B” to syringe “A” as heating continues.
- Push all the air slowly from syringe “A” back to syringe “B” and repeatedly back and forth.
- After about ten minutes, determine the new volume of air in syringe “B” V2
Set up of apparatus
Sample observations
Colour change from brown to black
Volume of air in syringe “B” before heating V1 = 158.0cm3
Volume of air in syringe “B” after heating V2 = 127.2cm3
Volume of air in syringe “B” used by copper V1 - V2 = 30.8cm3
Sample questions
- What is the purpose of:
- glass/cotton wool
To prevent/stop copper turnings from being blown into the syringe/out of the glass tube - Passing air through the glass tube repeatedly
To ensure all the active part of air is used up - Passing air through the glass tube slowly
To allow enough time of contact between the active part of and the heated copper turnings
- glass/cotton wool
- State and explain the observations made in the glass tube.
Colour change from brown to black
Brown copper metal reacts with the active part of air/oxygen to form black copper (II) oxide.
Chemical equation
Copper + Oxygen → Copper (II) oxide
2Cu(s) + O 2 (g) → 2CuO(s)
The reaction reduces the amount/volume of oxygen in syringe “B” leaving the inactive part of air. Copper only react with oxygen when heated. - Calculate the % of
- Active part of air
% active part of air = (V1 - V2) x 100% → 30.8cm3 x 100% = 19.493 % - Inactive part of air
Method 1
% inactive part of air = V2 x 100% →127.2cm3 x 100% = 80.506 %
Method 2
% inactive part of air = 100% -% active part of air
→ 100% - 19.493 % = 80.507 %
- Active part of air
- The % of active part of air is theoretically higher than the above while % of inactive part of air is theoretically lower than the above. Explain.
Not all the active part of air reacted with copper - State the main gases that constitute:
- active part of air.
Oxygen - Inactive part of air
Nitrogen, carbon (IV) oxide and noble gases
- active part of air.
- If the copper turnings are replaced with magnesium shavings the % of active part of air obtained is extraordinary very high. Explain.
Magnesium is more reactive than copper. The reaction is highly exothermic. It generates enough heat for magnesium to react with both oxygen and nitrogen in the air.
A white solid/ash mixture of Magnesium oxide and Magnesium nitride is formed. This considerably reduces the volume of air left after the experiment.Chemical equation
Magnesium + Oxygen → magnesium (II) oxide
2Mg(s) + O2 (g) → 2MgO(s)
Magnesium + Nitrogen -> magnesium (II) nitride
3Mg(s) + N2 (g) → Mg3N2 (s)
To find the composition of active part of air using alkaline pyrogallol
Procedure
- Measure about 2cm3 of dilute sodium hydroxide into a graduated gas jar.
- Record the volume of the graduated cylinder V1.
- Place about two spatula end full of pyrogallol/1, 2, 3-trihydroxobenzene into the gas jar.
- Immediately place a cover slip firmly on the mouth of the gas jar.
- Swirl thoroughly for about two minutes.
- Invert the gas jar in a trough/basin containing water.
- Measure the volume of air in the gas jar V2
Sample observations
Colour of pyrogallol/1, 2, 3-trihydroxobenzene change to brown.
Level of water in gas jar rises when inverted in basin/trough.
Volume of gas jar /air in gas jar V1= 800cm3
Volume of gas jar /air in gas jar after shaking with alkaline pyrogallol/1, 2, 3-trihydroxobenzene V2 = 640 cm3
Sample questions
- Which gas is absorbed by alkaline pyrogallol/1,2,3-trihydroxobenzene
Oxygen - Calculate the
- % of active part of air
(V1 -V2) x 100% → (800cm3 - 640 cm3) x 100% = 20% - % of inactive part of air
V2 x 100% → 640 cm3 x 100% = 80%
- % of active part of air
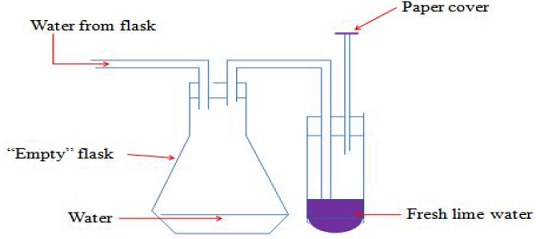
To establish the presence of carbon (IV) oxide in air using lime water
Pass tap water slowly into an empty flask as in the set up below.
Sample observation questions
- What is the purpose of paper cover?
To ensure no air enters into the lime water. - What happens when water enters the flask?
It forces the air from the flask into the lime water. - What is observed when the air is bubbled in the lime water?
A white precipitate is formed. The white precipitate dissolves on prolonged bubbling of air. -
- Identify the compound that form:
- lime water
Calcium hydroxide / Ca(OH)2 - White precipitate
Calcium carbonate/ CaCO3 - When the white precipitate dissolves
Calcium hydrogen carbonate/ CaHCO3
- lime water
- Write the chemical equation for the reaction that tale place when:
- White precipitate is formed
Calcium hydroxide + carbon (IV) oxide → Calcium carbonate + water
Ca(OH)2 (aq) + CO2 (g) → CaCO3 (s) + H2O (l) - White precipitate dissolves
Calcium carbonate + water + carbon (IV) oxide → Calcium hydrogen carbonate
CaCO3 (s) + H2O (l) + CO2 (g) → CaHCO3 (aq)
- White precipitate is formed
- Identify the compound that form:
- State the chemical test for the presence of carbon (IV) oxide gas based on 4(a) and (b) above:
Carbon (IV) oxide forms a white precipitate with lime water that dissolves in excess of the gas. - State the composition of carbon (IV) oxide gas by volume in the air.
About 0.03% by volume



Oxygen
Occurrence
Fifty 50% of the earth’s crust consist of Oxygen combined with other elements e.g. oxides of metals
About 70% of the earth is water made up of Hydrogen and Oxygen.
About 20% by volume of the atmospheric gases is Oxygen that form the active part of air.
School laboratory preparation.
Oxygen was first prepared in 1772 by Karl Scheele and later in 1774 by Joseph Priestly. It was Antony Lavoisier who gave it the name “Oxygen”
Procedure
Method 1: Using Hydrogen peroxide
- Half fill a trough/basin with tap water.
- Place a bee hive shelf/stand into the water.
- Completely fill the gas jar with water and invert in onto the bee hive shelf/stand.
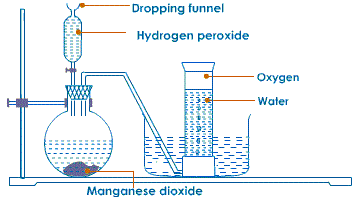
- Clamp a round bottomed flask and set up the apparatus as below.
- Collect several gas jars of Oxygen covering each sample.
Sample observation questions
- What is observed when the hydrogen peroxide is added into the flask?
Rapid effervescence/bubbling/fizzing - Describe the colour and smell of the gas
Colourless and odorless - Name the method of gas collection used.
- Over water
-Upward delivery
-Down ward displacement of water - What property of Oxygen makes it to be collected using the method above?
-Slightly soluble in water
- Name the method of gas collection used.
- What is the purpose of manganese (IV) oxide?
Manganese (IV) oxide is catalyst.
A catalyst is a substance that speeds up the rate of a chemical reaction but remain chemically unchanged at the end of the reaction. Hydrogen peroxide decomposes slowly to form water and Oxygen gas. A little Manganese (IV) oxide speeds up the rate of decomposition by reducing the time taken for a given volume of Oxygen to be produced. - Write the equation for the reaction.
Hydrogen peroxide → Water + Oxygen
2H2O2 (aq) → 2H2O (l) + O2 (g) - Lower a glowing splint slowly into a gas jar containing Oxygen gas. State what is observed.
The glowing splint relights/rekindles
Oxygen relights/rekindles a glowing splint. This is the confirmatory test for the presence of Oxygen gas
Method 2: Using Sodium peroxide
- Half fill a trough/basin with tap water.
- Add four drops of phenolphthalein indicator.
- Place a bee hive shelf/stand into the water.
- Completely fill a gas jar with water and invert in onto the bee hive shelf/stand.
- Clamp a round bottomed flask and set up the apparatus as below.
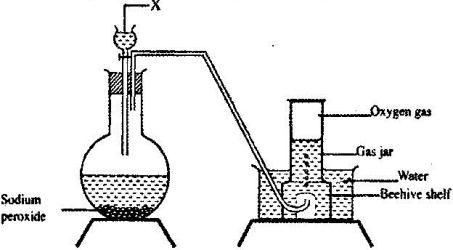
- Collect several gas jars of Oxygen covering each sample.
Sample observation questions
- What is observed when water is added?
- Into the flask containing sodium peroxide
Rapid effervescence/bubbling/fizzing - Phenolphththalein
Remains colourless /Phenolphthalein indicator is colourless in neutral solution
- Into the flask containing sodium peroxide
- Describe the colour and smell of the gas
Colourless and odorless - Name the method of gas collection used.
- Over water. Oxygen is slightly soluble in water. - Test the gas by lowering a glowing splint slowly into a gas jar containing the prepared sample.
The glowing splint relights/rekindles. This confirms the presence of Oxygen gas - Write the equation for the reaction.
Sodium peroxide + Water → Sodium hydroxide + Oxygen
2Na2O2 (aq) + 2H2O (l) → 4NaOH (aq) + O2 (g)
Method 3: Using Potassium Chlorate(V)
- Test the gas by lowering a glowing splint slowly into a gas jar containing the prepared sample.
The glowing splint relights/rekindles.
This confirms the presence of Oxygen gas - Write the equation for the reaction.
Potassium Chlorate (V) → Potassium Chloride + Oxygen
2KClO3 (aq) → 2KCl (aq) + 3O2 (g) - What is the purpose of manganese (IV) oxide?
Manganese (IV) oxide is catalyst .
A catalyst is a substance that speeds up the rate of a chemical reaction but remain chemically unchanged at the end of the reaction.
Potassium Chlorate (V) decomposes slowly to form potassium chloride and Oxygen gas.
A little Manganese (IV) oxide speeds up the rate of decomposition by reducing the time taken for a given volume of Oxygen to be produced.
Uses of Oxygen
- Oxygen is put in cylinders for use where natural supply is not sufficiently enough. This is mainly in:
- Mountain climbing/Mountaineering-at high altitudes, the concentration of air/oxygen is low. Mountain climbers must therefore carry their own supply of oxygen for breathing.
- Deep sea diving-Deep sea divers carry their own supply of Oxygen.
- Saving life in hospitals for patients with breathing problems and during anesthesia.
- A mixture of oxygen and some other gases produces a flame that is very hot.
- Oxy-acetylene/ethyne flame is produced when Ethyne/acetylene gas is burnt in pure oxygen. The flame has a temperature of about 3000oC.It is used for welding /cutting metals.
- Oxy-hydrogen flame is produced when Hydrogen is burn in pure oxygen. The flame has a temperature of about 2000oC.It is used also for welding /cutting metals.
- Oxy-hydrogen mixture is used as rocket fuel
- A mixture of charcoal, petrol and liquid Oxygen is an explosive.

Chemical properties of Oxygen /combustion
Oxygen is a very reactive non metal. Many elements react with oxygen through burning to form a group of compounds called Oxides.
Burning/combustion is the reaction of Oxygen with an element/substances.
Reaction in which a substance is added oxygen is called Oxidation reaction.
Burning/combustion are an example of an oxidation reaction.
Most non metals burn in Oxygen/air to form an Oxide which in solution / dissolved in water is acidic in nature.
They turn blue litmus red.e.g. Carbon (IV) oxide/CO2 , Nitrogen (IV) oxide/ NO2 , Sulphur (IV) oxide/ SO2
Some non metals burn in Oxygen/air to form an Oxide which in solution / dissolved in water is neutral in nature.
They don’t turn blue or red litmus. E.g. Carbon (II) oxide/CO, Water/ H2O
All metals burns in Oxygen/air to form an Oxide which in solution/dissolved in water is basic/alkaline in nature.
They turn red litmus blue.e.g.
Magnesium oxide/MgO, Sodium Oxide/ Na2O, Copper (II) oxide/CuO
Elements/substances burn faster in pure Oxygen than in air.
Air contains the inactive part of air that slows the rate of burning of substances/elements.
Reaction of metals with Oxygen/air
The following experiments show the reaction of metals with Oxygen and air.
Burning Magnesium
Procedure
- Cut a 2cm length piece of magnesium ribbon. Using a pair of tongs introduce it to a Bunsen flame. Remove it when it catches fire. Observe.
Place the products in a beaker containing about 5cm3 of water. Test the solution/mixture using litmus papers - Cut another 2cm length piece of magnesium ribbon. Using a pair of tongs introduce it to a Bunsen flame.
When it catches fire, lower it slowly into a gas jar containing Oxygen.
Place about 5cm3 of water into the gas jar. Test the solution/mixture using litmus papers. Test the solution/mixture using litmus papers
Observations
- In air
Magnesium burns with a bright blinding flame in air forming white solid/ash /powder. Effervescence/bubbles/fizzing Pungent smell of urine. Blue litmus paper remains blue. Red litmus paper turns blue - In pure Oxygen
Magnesium burns faster with a very bright blinding flame pure oxygen forming white solid/ash /powder. No effervescence/bubbles/ fizzing. No pungent smell of urine. Blue litmus paper remains blue. Red litmus paper turns blue.
Explanation
Magnesium burns in air producing enough heat energy to react with both Oxygen and Nitrogen to form Magnesium Oxide and Magnesium nitride. Both Magnesium Oxide and Magnesium nitride are white solid/ash/powder.
Chemical equations
Magnesium + Oxygen → Magnesium Oxide
2Mg(s) + O 2 (g) → 2MgO(s)
Magnesium + Nitrogen → Magnesium Nitride
3Mg(s) + N2 (g) → Mg3N2 (s)
Magnesium Oxide dissolves in water to form a basic/alkaline solution of Magnesium hydroxide.
Chemical equations
Magnesium Oxide + Water → Magnesium hydroxide
2Mg(s) + O2 (l) → 2MgO(s)
Magnesium Nitride dissolves in water to form a basic/alkaline solution of Magnesium hydroxide and producing Ammonia gas. Ammonia is also an alkaline/basic gas that has a pungent smell of urine.
Chemical equations
Magnesium Nitride + Water → Magnesium hydroxide + Ammonia gas
Mg3N2 (s) + 6H2O (l) → 3Mg(OH)2 (aq) + 2NH3 (g)
Burning Sodium
Procedure
- Carefully cut a very small piece of sodium. Using a deflagrating spoon introduce it to a Bunsen flame.
Remove it when it catches fire. Observe.
Place the products in a beaker containing about 20cm3 of water. Test the solution/mixture using litmus papers - Carefully cut another very small piece of sodium. Using a deflagrating spoon introduce it to a Bunsen flame.
When it catches fire, lower it slowly into a gas jar containing Oxygen.
Place about 20 cm3 of water into the gas jar. Test the solution/mixture using litmus papers. Test the solution/mixture using litmus papers
Observations
- In air
Sodium burns with a yellow flame in air forming a black solid. Blue litmus paper remains blue. Red litmus paper turns blue - In pure Oxygen
Sodium burns faster with a golden yellow flame in pure oxygen forming a yellow solid. Effervescence/bubbles/fizzing. Gas produced relights glowing splint. Blue litmus paper remains blue. Red litmus paper turns blue.
Explanation
- Sodium burns in air forming black Sodium Oxide
Chemical equations
Sodium + Oxygen/air → Sodium Oxide
4Na(s) + O2 (g) → 2Na2O(s)
Sodium Oxide dissolves in water to form a basic/alkaline solution of Sodium hydroxideChemical equations
Sodium Oxide + Water → Sodium hydroxide
Na2O(s) + H2O (l) → 2NaOH (aq) - Sodium burns in pure oxygen forming yellow Sodium peroxide
Chemical equations
Sodium + Oxygen → Sodium peroxide
2Na(s) + O2 (g) → Na2O2 (s)Sodium peroxide dissolves in water to form a basic/alkaline solution of Sodium hydroxide. Oxygen is produced.
Chemical equationsSodium Oxide + Water → Sodium hydroxide + Oxygen
2Na2O2 (s) + 2H2O (l) → 4NaOH ( aq ) + O2 (l)
Burning Calcium
Procedure
- Using a pair of tongs hold the piece of calcium on a bunsen flame.
Observe.
Place the products in a beaker containing about 2cm3 of water.
Test the solution/mixture using litmus papers. - Using a pair of tongs hold another piece of calcium on a Bunsen flame.
Quickly lower it into a gas jar containing Oxygen gas.
Observe.
Place about 2cm3 of water. Swirl.
Test the solution/mixture using litmus papers
Observations
- In air
Calcium burns with difficulty producing a faint red flame in air forming a white solid. Blue litmus paper remains blue. Red litmus paper turns blue - In pure Oxygen
Calcium burns with difficulty producing a less faint red flame Oxygen forming a white solid. Blue litmus paper remains blue. Red litmus paper turns blue
Explanation
Calcium burns in air forming white calcium Oxide. Calcium Oxide coat/cover the calcium preventing further burning.
Chemical equations
Calcium + Oxygen/air → calcium Oxide
2Ca(s) + O2 (g) → 2CaO(s)
Small amount of Calcium Oxide dissolves in water to form a basic/alkaline solution of Calcium hydroxide. The common name of Calcium hydroxide is lime water.
Chemical equations
Calcium Oxide + Water → Calcium hydroxide
CaO(s) + H2O (l) → Ca(OH)2 (aq)
Burning Iron
Procedure
- Using a pair of tongs hold the piece of Iron wool/steel wire on a Bunsen flame.
Observe.
Place the products in a beaker containing about 2cm3 of water.
Test the solution/mixture using litmus papers - Using a pair of tongs hold another piece of Iron wool/steel wire on a Bunsen flame.
Quickly lower it into a gas jar containing Oxygen gas .Observe.
Place about 2cm3 of water. Swirl. Test the solution/mixture using litmus papers
Observations
- In air
Iron wool/steel wire burns producing an Orange flame in air forming a brown solid. Blue litmus paper remains blue. Red litmus paper turns faint blue - In pure Oxygen
Iron wool/steel wire burns producing a golden Orange flame in Oxygen forming a Brown solid. Blue litmus paper remains blue. Red litmus paper turns faint blue
Explanation
- Iron burns in air forming brown Iron (III) Oxide
Chemical equations
Iron + Oxygen/air → Iron (III) Oxide
4Fe(s) + 3O2 (g) → 2Fe2O3 (s)
Very small amount of Iron (III) Oxide dissolves in water to form a weakly basic/alkaline brown solution of Iron (III) hydroxide.Chemical equations
Calcium Oxide + Water → Iron (III) hydroxide
Fe2O3 (s) + 3H2O (l) → 2Fe(OH)3 ( s )
Burning Copper
Procedure
- Using a pair of tongs hold the piece of copper turnings/shavings on a Bunsen flame.
Observe.
Place the products in a beaker containing about 2cm3 of water.
Test the solution/mixture using litmus papers - Using a pair of tongs hold another piece of Copper turnings/shavings on a Bunsen flame. Quickly lower it into a gas jar containing Oxygen gas.
Observe.
Place about 2cm3 of water. Swirl. Test the solution/mixture using litmus papers
Observations
- In air
Copper turnings/shavings burns with difficulty producing a green flame in air forming a black solid. Blue litmus paper remains blue. Red litmus paper turns faint blue - In pure Oxygen
Copper turnings/shavings burns less difficulty producing a green flame in Oxygen forming a Brown solid. Blue litmus paper remains blue. Red litmus paper turns faint blue
Explanation
Copper burns in air forming black Copper (II) Oxide
Chemical equations
Copper + Oxygen/air → Copper (II) Oxide
2 Cu(s) + O2 (g) → 2CuO(s)
Very small amount of Copper (II) Oxide dissolves in water to form a weakly basic/alkaline blue solution of Copper (II) hydroxide.
Chemical equations
Copper (II) Oxide + Water → Copper (II) hydroxide
CuO(s) + H2O (l) → Cu(OH)2(s)
Reaction of non metals with Oxygen/air
The following experiments show the reaction of non metals with Oxygen and air.
Burning Carbon
Procedure
- Using a pair of tongs hold a dry piece of charcoal on a Bunsen flame. Observe.
Place the products in a beaker containing about 2cm3 of water.
Test the solution/mixture using litmus papers. - Using a pair of tongs hold another piece of dry charcoal on a Bunsen flame. Quickly lower it into a gas jar containing Oxygen gas. Observe.
Place about 2cm3 of water. Swirl. Test the solution/mixture using litmus papers.
Observations
- Carbon chars then burns with a blue flame
- Colourless and odorless gas produced
- Solution formed turn blue litmus paper faint red.
- Red litmus paper remains red.
Explanation
Carbon burns in air and faster in Oxygen with a blue non-sooty/non-smoky flame forming Carbon (IV) oxide gas.
Carbon burns in limited supply of air with a blue non-sooty/non-smoky flame forming Carbon (IV) oxide gas.
Carbon (IV) oxide gas dissolves in water to form weak acidic solution of Carbonic (IV) acid.
Chemical Equation
Carbon + Oxygen → Carbon (IV) oxide
(excess air/oxygen)
C(s) + O2 (g) → CO2 (g) (in excess air)
Carbon + Oxygen → Carbon (II) oxide
(limited air/oxygen)
2C(s) + O2 (g) → 2CO (g) (in limited air)
Carbon (IV) oxide + Water → Carbonic (IV) acid
CO2 (g) + H2O (l) → H2CO3 (aq) (very weak acid)
Burning Sulphur
Procedure
- Using a deflagrating spoon place sulphur powder on a Bunsen flame.
Observe.
Place the products in a beaker containing about 3cm3 of water. Test the solution/mixture using litmus papers - Using a deflagrating spoon place sulphur powder on a Bunsen flame. Slowly lower it into a gas jar containing
Oxygen gas. Observe.
Place about 5cm3 of water. Swirl. Test the solution/mixture using litmus papers.
Observations
-Sulphur burns with a blue flame
-Gas produced that has pungent choking smell
-Solution formed turn blue litmus paper faint red.
Red litmus paper remains red.
Explanation
Sulphur burns in air and faster in Oxygen with a blue non-sooty/non-smoky flame forming Sulphur (IV) oxide gas.
Sulphur (IV) oxide gas dissolves in water to form weak acidic solution of Sulphuric (IV) acid.
Chemical Equation
Sulphur + Oxygen → Sulphur (IV) oxide
S(s) + O2 (g) → SO2 (g)(in excess air)
Sulphur (IV) oxide + Water → Sulphuric (IV) acid
SO2 (g) + H2O (l) → H2SO3 (aq) (very weak acid)
Burning Phosphorus
Procedure
- Remove a small piece of phosphorus from water and using a deflagrating spoon (with a lid cover) places it on a Bunsen flame.
Observe.
Carefully put the burning phosphorus to cover gas jar containing about 3cm3 of water. Test the solution/mixture using litmus papers - Remove another small piece of phosphorus from water and using a deflagrating spoon (with a lid cover) place it on a Bunsen flame.
Slowly lower it into a gas jar containing Oxygen gas with about 5 cm3 of water. Observe.
Swirl. Test the solution/mixture using litmus papers.
Observations
- Phosphorus catches fire before heating on Bunsen flame
- Dense white fumes of a gas produced that has pungent choking poisonous smell
- Solution formed turn blue litmus paper faint red. Red litmus paper remains red.
Explanation
Phosphorus is stored in water. On exposure to air it instantaneously fumes then catch fire to burn in air and faster in Oxygen with a yellow flame producing dense white acidic fumes of Phosphorus (V) oxide gas.
Phosphoric (V) oxide gas dissolves in water to form weak acidic solution of Phosphoric (V) acid.
Chemical Equation
Phosphorus + Oxygen → Phosphorous (V) oxide
4P(s) + 5O2 (g) → 2P2O5 (s)
Phosphorous (V) oxide + Water → Phosphoric (V) acid
P2O5 (s) + 3H2O (l) → 2H3PO4 (aq) (very weak acid)
Reactivity series/competition for combined Oxygen.
- The reactivity series is a list of elements/metals according to their affinity for oxygen. Some metals have higher affinity for Oxygen than others.
- A metal/element with higher affinity for oxygen is placed higher/on top of the one less affinity.
The complete reactivity series of metals/elements
| Element/Metal | Symbol |
| Potassium | K |
| Sodium | Na |
| Calcium | Ca |
| Magnesium | Mg |
| Aluminum | Al |
| Carbon | C |
| Zinc | Zn |
| Iron | Fe |
| Tin | Sn |
| Lead | Pb |
| Hydrogen | H |
| Copper | Cu |
| Mercury | Hg |
| Silver | Ag |
| Gold | Au |
| Platinum | Pt |
Metals compete for combined Oxygen. A metal/element with higher affinity for oxygen removes Oxygen from a metal lower in the reactivity series/less affinity for Oxygen.
When a metal/element gains/acquire Oxygen, the process is called Oxidation.
When metal/element donate/lose Oxygen, the process is called Reduction.
An element/metal/compound that undergoes Oxidation is called Reducing agent.
An element/metal/compound that undergoes Reduction is called Oxidizing agent.
A reaction in which both Oxidation and Reduction take place is called a Redox reaction.
Redox reaction between Magnesium and copper (II) Oxide
Procedure
- Place about 2g of copper (II) oxide in a crucible with a lid.
- Place another 2g of Magnesium powder into the crucible.
- Mix thoroughly.
- Cover the crucible with lid.
- Heat strongly for five minutes.
- Allow the mixture to cool.
- Open the lid.
- Observe.
Observation
Colour change from black to brown. White solid power formed.
Explanation
Magnesium is higher in the reactivity series than Copper. It has therefore higher affinity for Oxygen than copper.
When a mixture of copper (II) oxide and Magnesium is heated, Magnesium reduces copper (II) oxide to brown copper metal and itself oxidized to Magnesium oxide.
Magnesium is the reducing agent because it undergoes oxidation process.
Copper (II) oxide is the oxidizing agent because it undergoes redox reduction process.
The mixture should be cooled before opening the lid to prevent hot brown copper from being reoxidized back to black copper (II) oxide.
Chemical equation
- Copper (II) oxide + Magnesium → Magnesium oxide + Copper
CuO (s) + Mg(s) → MgO (s) + Cu (s) - Zinc (II) oxide + Magnesium → Magnesium oxide + Zinc
ZnO (s) + Mg(s) → MgO (s) + Zn (s) - Zinc (II) oxide + Carbon → Carbon (IV) oxide gas + Zinc
ZnO (s) + C(s) → CO2 (g) + Zn (s)
The reactivity series is used during extraction of metals from their ore. An ore is a rock containing mineral element which can be extracted for commercial purposes. Most metallic ores occur naturally as:
- oxides combined with Oxygen
- sulphides combined with Sulphur
- carbonates combined with carbon and Oxygen.
Metallic ores that naturally occur as metallic sulphides are first roasted in air to form the corresponding oxide.
Sulphur (IV) oxide gas is produced. e.g.
Copper (I) sulphide + Oxygen → Copper (I) Oxide + Sulphur (IV) oxide
Cu2S(s) + O2 (g) → 2Cu(s) + SO2 (g)
Zinc (II) sulphide + Oxygen → Zinc (II) Oxide + Sulphur (IV) oxide
ZnS(s) + O2 (g) → Zn(s) + SO2 (g)
Lead (II) sulphide + Oxygen → Lead (II) Oxide + Sulphur (IV) oxide
PbS(s) + O2 (g) → Pb(s) + SO2 (g)
Iron (II) sulphide + Oxygen → Iron (II) Oxide + Sulphur (IV) oxide
FeS(s) + O2 (g) → Fe(s) + SO2 (g)
Metallic ores that naturally occur as metallic carbonates are first heated in air. They decompose /split to form the corresponding oxide and produce Carbon (IV) oxide gas. .e.g.
Copper (II) carbonate → Copper (II) oxide + Carbon (IV) oxide
CuCO3 (s) → CuO(s) + CO2 (g)
Zinc (II) carbonate → Zinc (II) oxide + Carbon (IV) oxide
ZnCO3 (s) → ZnO(s) + CO2 (g)
Lead (II) carbonate → Lead (II) oxide + Carbon (IV) oxide
PbCO3 (s) → PbO(s) + CO2 (g)
Iron (II) carbonate → Iron (II) oxide + Carbon (IV) oxide
FeCO3 (s) → FeO(s) + CO2 (g)
Download AIR AND COMBUSTION - Chemistry Notes Form 1.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students