- Introduction
- Conductors and Insulators
- Electrolytes and Non-electrolytes
- Ionisation
- Electrolytic Dissociation
- Electrolysis of a Compound
- Metallic Conductivity
- Electrolytic Conductivity
- Electrolysis Circuit
- Factors that Determine Products of Electrolysis
- Electrolysis of Dilute Sulphuric Acid (Electrolysis of Water)
- Electrolysis of Copper(II)Sulphate Solution
- Electroplating
- Electrolysis of Lead Bromide.

Introduction
- In any chemical reaction, the existing chemical bonds are broken and new chemical bonds are formed.
- Hence, all chemical reactions are fundamentally electrical in nature since electrons are involved in some way or the other in all types of chemical bonding.
- Many chemical reactions utilize electrical energy, whereas others can be used to produce electrical energy.
- As electrical energy involves the flow of electrons, these reactions are concerned with the transfer of electrons from one substance to the other.

Conductors and Insulators
- The ability to conduct electricity is the major simple distinction between elements that are metals and non-metals.
Conductors
- A conductor is a material that conducts electricity but is not chemically changed in the process.
- All metals and graphite are conductors of electricity.
Insulators
- An insulator is a material that does not conduct electricity. Such materials have no free electrons.
Summary of Common Electrical Conductors
These materials carry an electric current via freely moving electrically charged particles, when a potential difference (voltage) is applied across them, and they include:
- All metals (molten or solid) and the non-metal carbon (graphite).
- This conduction involves the movement of free or delocalised electrons (e- charged particles) and does not involve any chemical change. - Any molten or dissolved material in which the liquid contains free moving ions is called the electrolyte. Ions are charged particles e.g. Na+ sodium ion, or Cl- chloride ion, and their movement or flow constitutes an electric current, because a current is moving charged particles.
- The movement of opposite charges during electrolysis is due to the attracting in the electric field produced by the potential difference (the voltage).
- Liquids that conduct must contain freely moving ions to carry the current and complete the circuit.
- You can't do electrolysis with an ionic solid! The ions are too tightly held by chemical bonds and can't flow from their ordered situation!
- When ionically bonded substances are melted or dissolved in water the ions are free to move about. However some covalent substances dissolve in water and form ions. e.g. hydrogen chloride HCl, dissolves in water to form 'ionic' hydrochloric acid H+Cl-(aq).

Electrolytes and Non-electrolytes
- An ionic or electrovalent compound that conducts electricity in molten (fused) or aqueous (solution) state can be classified as an electrolyte.
- However, if the compound is unable to ionise it does not conduct electricity it is called a non-electrolyte.
- In general, the extent to which an electrolyte can break up into ions categorises an electrolyte. This gives a measure of the degree of dissociation (a) of an electrolyte. Based on this degree the electrolytes can be classified as strong or weak electrolyte and non-electrolyte.
Strong Electrolyte
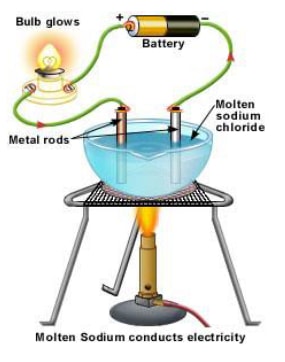
- A strong electrolyte, such as a solution of sodium chloride dissociates or ionises completely or almost completely to form free mobile ions in the solution or molten form.
- The more the availability of free mobile ions in an electrolyte, the greater is its capacity to carry or conduct current i.e. the stronger the electrolyte.
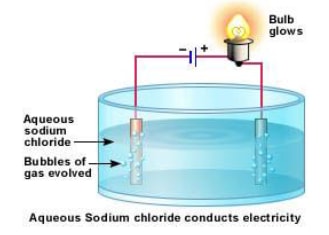
- The ability to conduct current can be observed by setting up a cell as shown in figure below. The bulb glows brightly.
- For e.g., Sodium chloride even in crystalline form consists of ions. But the ions are not mobile so it does not conduct electricity and the bulb does not light. When melted or dissolved in water, it dissociates completely into free, mobile ions.
NaCl(aq) ⇌ Na+(aq) + Cl-(aq) - Pure sulphuric acid exists mostly in the form of molecules. But when mixed with water, it almost completely breaks up into free mobile ions.
H2SO4(l) ⇌ 2H+(aq) + SO42-(aq)
Weak Electrolyte
- A weak electrolyte ionises or dissociates only partially to form free mobile ions.
- Most of the electrolyte remains as un-ionised molecules. For example in acetic acid, the number of its dissociated ions (the acetate and hydrogen ions) is less compared to the total amount of acetic acid molecules present.
- Similarly in ammonium hydroxide the number of its dissociated ions (the ammonium and hydroxyl ions) are less compared to the total amount of the molecules present.
CH3COOH(aq) ⇌ CH3COO-(aq) + H+(aq)
NH4OH(aq) ⇌ NH4+(aq) + OH-(aq)
Thus both these compounds are weak electrolytes. - When the number of mobile ions is less in an electrolyte, the lesser is its capacity to carry or conduct current i.e. the weaker is the electrolyte.
- If one liter of a solution containing one molar mass of sulphuric acid, and one liter of a solution containing one molar mass of citric or acetic acid, are subjected to the same current, then:
- The bulb glows brightly in the case of the sulphuric acid, showing it to be a strong electrolyte
- The bulb glows dimly in the case of the citric or acetic acid, showing that it is a weak electrolyte.
Non-electrolyte
- A non-electrolyte does not provide ions in a solution and therefore current does not flow through such solution.
- Some examples of non-electrolytes are: alcohol, carbon tetrachloride, carbon disulphide.
Examples of Electrolytes
| Strong electrolyte | Weak electrolytes | Non- electrolytes |
| Sea water | Tap water | Pure water |
| Dil.sulphuric acid | Carbonic acid | Paraffin |
| Dil.nitric acid | Ammonium hydroxide | alcohol |
| Dil.hydrochloric acid | Oxalic acid | Aqueous sugar solution |
| Aqueous sodium chloride | Ethanoic acid | |
| Aqueous potassium hydroxide | ||
| Aqueous sodium hydroxide | ||
| Molten lead bromide |

Ionisation
- 'The process of conversion of a neutral atom into charged ions to complete its octet is known as ionization.'
- In this process, the neutral atom loses or gains electrons.
- The particle that loses electrons gains positive charge equal to the number of electrons lost, while the particle that gains electrons gains negative charge equal to the number of electrons gained.
- When atoms from metallic elements combine with those from non-metals, they do so by transfer of electrons from one atom to another, forming compounds having "ionic or electrovalent" bonds.
- The neutral atom that loses an electron becomes a cation and the neutral atom that acquires an electron becomes an anion.
- For e.g., when a sodium atom combines with a chlorine atom to form sodium chloride, the sodium atom loses one electron and becomes positively charged ion.
- The chlorine atom gains the electron and it becomes negatively charged ion.

Electrolytic Dissociation
- Electrovalent substances are made up of ions in the solid state.
- The oppositely charged ions are held together by strong electrostatic force of attraction.
- Due to these forces the ions cannot move.
- However, when these substances are dissolved in water or melted, the ions free themselves from this binding.
- Thus the break up of an electrovalent compound into free mobile ions when dissolved in water or when melted, is called electrolytic dissociation.
Theory of Electrolytic Dissociation
- The main ideas of the ionic theory or theory of electrolytic dissociation are as follows:
- On dissolving in water an electrolyte, breaks up into free cations and anions. The energy associated with moving charges is called current or electricity.
- The ions carry an electric charge and also allow the flow of electric current through it. The flow of electricity is due to the flow of the ions.
- The total number of positive and negative charges of the ions in the compound is equal.

Electrolysis of a Compound
- When substances which are made of ions are dissolved in water, or melted material, they can be broken down (decomposed) into simpler substances by passing an electric current through them.
- This process is called electrolysis. Since it requires an 'input' of energy, it is an endothermic process.
During electrolysis:
- Positive metal ions or hydrogen ions move to the negative electrode where the metal lower in the reactivity series gets discharged by gain of electrons (a reduction process). They are known as cations because they drift towards the cathode.
- Negative non-metal ions drift to the positive electrode (anode) where again the less reactive ions get discharged from the solution by loss of electrons (oxidation). They are known as anions because they drift towards the anode.
- In the electrolyte (solution or melt of free moving ions), Positive metal or hydrogen ions move to the negative electrode (cations attracted to cathode), e.g. in the diagram, sodium ions Na+, move to the -ve electrode, and negatively charged ions move to the positive electrode (anions attracted to anode), e.g. in the diagram, chloride ions Cl-, move to the +ve electrode.
- During electrolysis, gases may be given off, or metals dissolve or are deposited at the electrodes.
In summary, the following substances are electrolytes:
- Molten salts
- Solutions of salts in water
- Solutions of acids
- Solutions of alkalis

Metallic Conductivity
- Electrons flow(carry charge)
- It is a property of elements, graphite and alloys
- It takes place in solids and liquids
- No chemical change takes place.

Electrolytic Conductivity
- Ions flow (carry charge)
- It is a property of ionic compounds
- Takes place in liquids (molten salts) and solutions but not solids
- Chemical decomposition takes place.

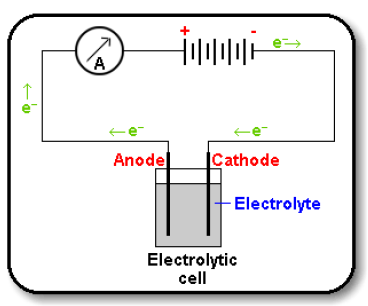
Electrolysis Circuit
- There are two ion movements in the electrolyte flowing in opposite directions.
- Positive cations e.g. Na+ attracted to the negative cathode electrode.
- Negative anions e.g. Cl- attracted to the positive anode electrode.
- No electrons flow in the solution.
- They only flow in metal wires or carbon (graphite) electrodes of the external circuit.
- The molten or dissolved materials (electrolytes) are usually acids, alkalis or salts and their electrical conduction is usually accompanied by chemical changes e.g. decomposition.
- Liquids that conduct must contain freely moving ions to carry the current and complete the circuit.
- Electrolysis can’t be performed with an ionic solid. This is because the ions are too tightly held by chemical bonds and can't flow.
- When ionically bonded substances are melted or dissolved in water, the ions are free to move about.
- However some covalent substances dissolve in water and form ions. Hydrogen chloride (HCl) is covalent.
- However it dissolves in water to form 'ionic' hydrochloric acid H+Cl-(aq)
Electrode Reactions
Cathode Reactions (Reduction)
- (-) negative cathode where reduction of the attracted positive cations is by electron gain (reduction) to form metal atoms or hydrogen [from Mn+ or H+, n = numerical charge].
- The electrons come from the positive anode.
- Hydrogen ions are reduced to hydrogen gas molecules.
- Electrolysis of many dilute salts or acid solutions make hydrogen gas by reduction as shown.
2H+(aq) + 2e- → H2(g) - Copper (II) ions are reduced to copper atoms in the electrolytic purification or electroplating using copper (II) sulphate solution.
Cu2+(aq) + 2e- → Cu(s) - Silver ions reduced to silver atoms in silver electroplating
Ag+(aq) + e- → Ag(s)
Anode Reactions (Oxidation)
- Positive anode is where the oxidation of the atom or anion is by electron loss.
- Non-metallic negative anions are attracted and may be oxidised to the free element.
- For example, in the electrolysis of molten chloride salts or their concentrated aqueous solution or conc. hydrochloric acid ,chloride ion oxidised to chlorine gas molecules.
2Cl-(l/aq) → Cl2(g) + 2e- - In the electrolysis of molten oxides eg anode reaction in the extraction of aluminium from molten bauxite, oxide ion oxidised to oxygen gas molecules.
2O2-(l) → O2(g) + 4e- - The electrons released by this process travel round the circuit and are donated to the cations (reduction).
- Electrolysis of many salt solutions such as sulphates, sulphuric acid etc. gives oxygen. Hydroxide ions oxidised to oxygen gas molecules.
4OH-(aq) → 2H2O(l) + O2(g) + 4e-


Factors that Determine Products of Electrolysis
- The ions that are successfully released (or discharged) at the electrodes depend on three factors:
- The position of the ion in the electrochemical series
- The concentration of the ion in the solution
- The nature of the electrode
The position of the ion in the electrochemical series
- This is probably better expressed as the position of the ions in the electrochemical series.
- The ions that are lower in the electrochemical series get discharged in preference to the ones above them.
- For e.g., if a solution has potassium ions and copper ions, the copper ions will accept electrons, and get discharged as copper atoms first. The potassium ions will not be affected.
Element Symbol Group Number Potassium K 1 Sodium Na 1 Calcium Ca 2 Magnesium Mg 2 Aluminium Al 3 Carbon C 4 (non metal) Zinc Zn Transition Metal Iron Fe Transition Metal Lead Pb 4 Hydrogen H Non metal Copper Cu Transition Metal Silver Ag Transition Metal Gold Au Transition Metal - As a rule, in a dilute solution, if the metal appears below hydrogen in the electrochemical series then it will be deposited preferentially.
The concentration of the ions
- When two ions with similar reactivity are in competition then the relative concentration of the two ions becomes an important factor. If an electrolyte contains a higher concentration of ions, which are higher in the electrochemical series than those that are lower, then these ions get discharged in preference to the lower ones.
- For e.g., a solution of sodium chloride in water contains two types of anions i.e., the chloride (Cl-) ions and the hydroxyl (OH-) ions. Hydroxyl ions are lower in the electrochemical series than chloride ions. But if the concentration of chloride ions is much higher than that of the hydroxyl ions then the chloride ions get discharged first.
Electrolysis of NaCl Solution at different Concentration
| Dilute Solution (aq) | Concentrated Solution (aq) |
| NaCl ⇌ Na+ + Cl- H2O ⇌ H+ + OH- |
NaCl ⇌ Na+ + Cl- H2O ⇌ H+ + OH- |
| Ions discharged At Cathode: H+ At Anode: OH- |
Ions discharged At Cathode: Na+ At Anode: Cl- (chloride) |
| Products At Cathode: Hydrogen At Anode: Oxygen [Discharge takes place according to electrochemical series] |
Products At Cathode: Sodium (forms NaOH) At Anode: Chlorine [Discharge is not according to the electrochemical series since the concentration of Na+ and Cl- is more than H+ and OH-] |
The nature of the electrode
- Usually inert electrodes such as graphite or platinum are used for electrolysis.
- These electrodes do not interfere with the reactions occurring at the surface of the electrode they simply act as a point of connection between the electrical circuit and the solution.
- However, if metal electrodes are used in same metal ion solutions, they can get involved in the reactions by dissolving as ions leaving their electrons behind.
Example: Electrolysis of sodium chloride solution
The ions present in the solution are:
Na+, Cl-, H+, OH-
At the cathode
- The positive ions are attracted to the negative cathode.
- There is competition between the sodium ions and the hydrogen ions.
- As the hydrogen ion hydrogen ion is lower in the electrochemical series than the sodium ion sodium, the hydrogen ions are preferentially reduced and hydrogen gas is produced at the electrode (bubbles are seen)
2H+ + 2e → H2
At the anode
- There is competition between the negative ions at the positive anode.
- The chloride ions compete with the hydroxide ions to release their electrons to the anode.
- If the solution is fairly concentrated the chloride ions preferentially lose electrons to become chlorine atoms (and then molecules).
2Cl- → Cl2 + 2e
Ions remaining in solution
- The ions that are removed from the solution, then, are the hydrogen ions and the chloride ions. This means that the sodium ions and the hydroxide ions remain in the solution - i.e. sodium hydroxide is also produced.
- Note: When the solution of chloride ions is dilute then OH, ions are preferentially released at the anode.

Electrolysis of Dilute Sulphuric Acid (Electrolysis of Water)
- Water is a poor conductor of electricity.
- However, it can be made to decompose if some dilute sulphuric acid is added.
- A Hofmann voltammeter below can be used to keep the gases produced separate.
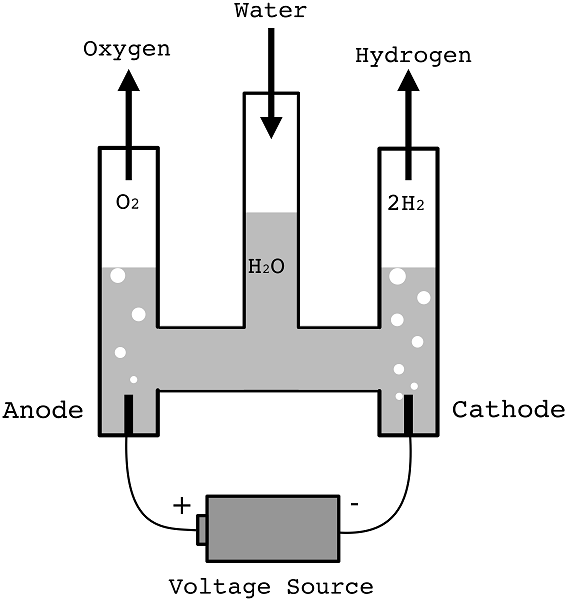
- At anode 4OH-(aq) → 2H2O(l) + O2(g) + 4e-
- At cathode 2H+(l) + 2e- → H2(g)
- After a while, the volume of gas in each arm can be measured and tested.
- Oxygen collects at the anode.
- The ratio of volumes is about 2:1 for hydrogen and oxygen respectively.
- Effectively, this experiment is the electrolysis of water.

Electrolysis of Copper(II)sulphate Solution
- The ions present in the solution are:
Cu2+, SO42-,H+, OH-
At the cathode
- The positive ions are attracted to the negative cathode.
- There is competition between the copper ions and the hydrogen ions.
- As the hydrogen ion appears higher in the electrochemical series than the copper ion, copper ions are preferentially reduced and copper metal is deposited at the electrode (pink layer is observed).
Cu2+ + 2e → Cu
At the anode
- There is competition between the negative ions at the anode.
- The sulphate ions compete with the hydroxide ions to release their electrons to the anode.
- The hydroxide ions are lower in the series and are preferentially released as oxygen gas (bubbles are seen) and water.
4OH- → 2H2O + O2 +4e
OILRIG
Oxidation Is Loss, Reduction Is Gain (of electrons)
Oxidising agents are easily reduced.
Reducing agents are easily oxidized.
Ions remaining in solution
- The ions that are removed from the solution, then, are the copper ions and the hydroxide ions, this means that the hydrogen ions and the sulphate ions remain in the solution - i.e. sulphuric acid is also produced.
- The solution changes colour from blue to colourless.

Electroplating
- Electroplating is a process of depositing a thin layer of a fine and superior metal (like chromium, zinc, nickel, gold etc.) over the article of a baser and cheaper metal (like iron, copper, brass), with the help of electric current.
Uses
Electroplating is very useful because of the following reasons:
- Surface protection e.g. nickel plating of iron to prevent corrosion.
- Makes the article attractive e.g., electroplating of silver or gold on brass etc.
- Repair of finer machine parts.
Process
The process of electroplating involves the following steps:
- Before electroplating the metal surface is cleaned thoroughly. Firstly, an alkaline solution is used to remove grease and then it is treated with acid to remove any oxide layer. It is then washed with water.
- The article to be electroplated is made cathode since metallic ions are positive and thus get deposited on the cathode.
- The anode is made of pure metal, which is to be coated on the article.
- The electrolyte is the salt of the metal to be coated on the article.
- A direct (D.C.) current is passed through the electrolyte. The anode dissolves, depositing the metal ions from the solution on the article in the form of a metallic coating. The passage of low current is continued for a long time to ensure an even coating.
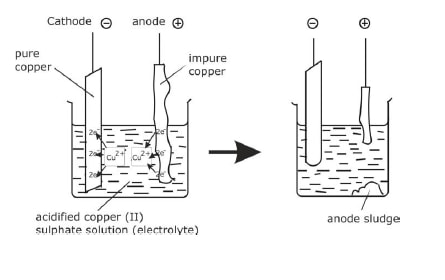
Electrolysis of Copper (II) sulphate Solution using Copper Electrodes
The ions present in the solution are:
Cu2+, SO42-,H+, OH-
At the cathode
- The positive ions are attracted to the negative cathode.
- There is competition between the copper ions and the hydrogen ions.
- As the hydrogen ions are higher in the electrochemical series, the copper ions are preferentially reduced and copper metal is deposited at the electrode (a pink layer is observed)
Cu2+ + 2e- → Cu
At the anode
- In this case the electrode is made of copper and it is easier for the copper to dissolve leaving its electrons behind on the anode than for any other ion to be released.
Cu → Cu2+ + 2e
Ions remaining in solution
- Copper is deposited at the cathode and is dissolved at the anode.
- Consequently the concentration of copper ions in solution remains constant.
- This can be used as a method of purification of copper as only pure copper is deposited at the cathode.
- In this purification an anode made of impure copper is turned to pure copper at the cathode leaving the impurities behind (the sludge in the diagram).
Basic rules for electroplating an object metal M are as follows:
- The object must be made the cathode
- The electrolyte must be a solution of a salt of metal M,
- The anode is made of a strip of metal M

Electrolysis of Lead Bromide.
- Lead bromide must be heated until it is molten before it will conduct electricity.
- Electrolysis separates the molten ionic compound into its elements.
- The reactions at each electrode are called half equations.
- The half equations are written so that the same number of electrons take part in each equation.
Pb2+ + 2e- → Pb (lead metal at the (-) cathode).
2Br- → Br2 + 2e- (bromine gas at the (+) anode). - Lead ions gain electrons (reduction) to form lead atoms. Bromide ions lose electrons (oxidation) to form bromine atoms. The bromine atoms combine to form molecules of bromine gas.
The overall reaction is;
PbBr2(l) → Pb(s) + Br2(g)
Summary
- Reactive metals (more reactive than hydrogen) are never deposited during electrolysis of aqueous solutions.
- If the metal ion comes from a metal more reactive than hydrogen then hydrogen gas is liberated at the cathode.
- Halide ions (chloride, bromide, and iodide) are released preferentially and if these are not present then the hydroxide ions from the water are released at the anode.
Download EFFECT OF AN ELECTRIC CURRENT ON SUBSTANCES - Chemistry Notes Form 2.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students