Introduction
- Carbon is an element in Group IV(Group 4)of the Periodic table.
- It has atomic number 6 and electronic configuration 2:4 and thus has four valence electrons(tetravalent).
- It does not easily ionize but forms strong covalent bonds with other elements including itself.
Occurrence
Carbon mainly naturally occurs as:
- allotropes of carbon i.e graphite, diamond and fullerenes.
- amorphous carbon in coal, peat ,charcoal and coke.
- carbon(IV)oxide gas accounting 0.03% by volume of normal air in the atmosphere.
Allotropes of Carbon
Carbon naturally occur in two main crystalline allotropic forms, carbon-graphite and carbon-diamond.
Differences between Carbon-diamond and Carbon-graphite
| Carbon-diamond | Carbon-graphite |
| Shiny crystalline solid | Black/dull crystalline solid |
| Has a very high melting/boiling point because it has a very closely packed giant tetrahedral structure joined by strong covalent bonds | Has a high melting/boiling point because it has a very closely packed giant hexagonal planar structure joined by strong covalent bonds |
| Has very high density(Hardest known natural substance) | Soft |
| Abrassive | Slippery |
| Poor electrical conductor because it has no free delocalized electrons | Good electrical conductor because it has free 4th valency delocalized electrons |
| Is used in making Jewels, drilling and cutting metals | Used in making Lead-pencils,electrodes in batteries and as a lubricant |
| Has giant tetrahedral structure | Has giant hexagonal planar structure |

Properties of Carbon
Physical Properties of Carbon
- Carbon occur widely and naturally as a black solid
- It is insoluble in water but soluble in carbon disulphide and organic solvents.
- It is a poor electrical and thermal conductor.
Chemical Properties of Carbon
1) Burning
Experiment
- Introduce a small piece of charcoal on a Bunsen flame then lower it into a gas jar containing Oxygen gas.
- Put three drops of water.
- Swirl.
- Test the solution with blue and red litmus papers.
Observation
- Carbon chars then burns with a blue flame
- Colourless and odourless gas produced
- Solution formed turn blue litmus paper faint red. Red litmus paper remains red.
Explanation
- Carbon burns in air and faster in Oxygen with a blue non-sooty/non-smoky flame forming Carbon (IV) oxide gas.
- Carbon burns in limited supply of air with a blue non-sooty/non-smoky flame forming Carbon (IV) oxide gas.
- Carbon (IV) oxide gas dissolve in water to form weak acidic solution of Carbonic (IV)acid.
Chemical Equation
C(s) + O2(g) → CO2(g) (in excess air)
2C(s) + O2(g) → 2CO(g) (in limited air)
CO2(g) + H2O(l) → H2CO3(aq) (very weak acid)
2). Reducing agent
Experiment
- Mix thoroughly equal amounts of powdered charcoal and copper (II)oxide into a crucible.
- Heat strongly.
Observation
- Colour change from black to brown
Explanation
- Carbon is a reducing agent.
- For ages it has been used to reducing metal oxide ores to metal, itself oxidized to carbon(IV)oxide gas.
- Carbon reduces black copper(II)oxide to brown copper metal
Chemical Equation
2CuO(s) + C(s) → 2Cu(s) + CO2(g)
2PbO(s) + C(s) → 2Pb(s) + CO2(g)
2ZnO(s) + C(s) → 2Zn(s) + CO2(g)
Fe2O3(s) + 3C(s) → 2Fe(s) + 3CO2(g)
Fe3O4(s) + 4C(s) → 3Fe(s) + 4CO2(g)

Compounds of Carbon
- The following are the main compounds of Carbon
- Carbon(IV)Oxide(CO2)
- Carbon(II)Oxide(CO)
- Carbonate(IV) (CO32-)and hydrogen carbonate(IV(HCO3-)
- Sodium carbonate(Na2CO3)
Carbon(IV)Oxide (CO2)
i) Occurrence
Carbon(IV)oxide is found:
- in the air /atmosphere as 0.03% by volume.
- a solid carbon(IV)oxide mineral in Esageri near Eldame Ravine and Kerita near Limuru in Kenya.
ii) School Laboratory Preparation
- In the school laboratory carbon(IV)oxide can be prepared in the school laboratory from the reaction of marble chips(CaCO3)or sodium hydrogen carbonate(NaHCO3) with dilute hydrochloric acid.
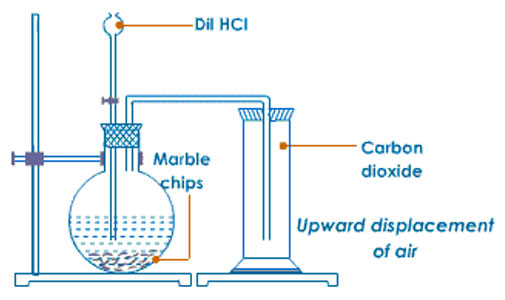
iii) Properties of Carbon(IV)oxide Gas(Questions)
- Write the equation for the reaction for the school laboratory preparation of carbon (IV)oxide gas.
Any carbonate reacted with dilute hydrochloric acid should be able to generate carbon (IV)oxide gas.
Chemical equations
CaCO3(s) + 2HCl(aq) → CaCO3(aq) + H2O(l) + CO2(g)
ZnCO3(s) + 2HCl(aq) → ZnCO3(aq) + H2O(l) + CO2(g)
MgCO3(s) + 2HCl(aq) → MgCO3(aq) + H2O(l) + CO2(g)
CuCO3(s) + 2HCl(aq) → CuCO3(aq) + H2O(l) + CO2(g)
NaHCO3(s) + HCl(aq) → Na2CO3(aq) + H2O(l) + CO2(g)
KHCO3(s) + HCl(aq) → K2CO3(aq) + H2O(l) + CO2(g) - What method of gas collection is used in preparation of Carbon(IV)oxide gas. Explain.
Downward delivery /upward displacement of air/over mercury
Carbon(IV)oxide gas is about 1½ times denser than air. - What is the purpose of:
- water? To absorb the more volatile hydrogen chloride fumes produced during the vigorous reaction.
- sodium hydrogen carbonate? To absorb the more volatile hydrogen chloride fumes produced during the vigorous reaction and by reacting with the acid to produce more carbon (IV)oxide gas.
Chemical equation
NaHCO3(s) + HCl(aq) → Na2CO3(aq) + H2O(l) + CO2(g)
- concentrated sulphuric(VI)acid?
To dry the gas/as a drying agent - Describe the smell of carbon(IV)oxide gas
Colourless and odourless - Effect on lime water.
Experiment
Bubble carbon(IV)oxide gas into a test tube containing lime water for about three minutes
Observation
White precipitate is formed.
White precipitate dissolved when excess carbon(IV)oxide gas is bubbled.
Explanation
Carbon(IV)oxide gas reacts with lime water(Ca(OH)2) to form an insoluble white precipitate of calcium carbonate. Calcium carbonate reacts with more Carbon(IV) oxide gas to form soluble Calcium hydrogen carbonate.
Chemical equation
Ca(OH)2(aq) + CO2(g) → CaCO3(s) + H2O(l)
CaCO3(aq) + H2O(l) + CO2(g) → Ca(HCO3)2(aq) - Effects on burning Magnesium ribbon
Experiment
Lower a piece of burning magnesium ribbon into a gas jar containing carbon (IV)oxide gas.
Observation
The ribbon continues to burn with difficulty
White ash/solid is formed.
Black speck/solid/particles formed on the side of gas jar.
Explanation
Carbon(IV)oxide gas does not support combustion/burning. Magnesium burn to produce/release enough heat energy to decompose Carbon(IV) oxide gas to carbon and oxygen.Magnesium continues to burn in Oxygen forming white Magnesium Oxide solid/ash.Black speck/particle of carbon/charcoal residue forms on the sides of reaction flask. During the reaction Carbon(IV) oxide is reduced(Oxidizing agent)to carbon while Magnesium is Oxidized to Magnesium Oxide.
Chemical equation
2Mg(s) + CO2(g) → C(s) + 2MgO(l) - Dry and wet litmus papers were separately put in a gas jar containing dry carbon (IV)oxide gas. State and explain the observations made.
Observation
Blue dry litmus paper remain blue
Red dry litmus paper remain Red
Blue wet/damp/moist litmus paper turn red
Red wet/damp/moist litmus paper remain red
Explanation
Dry Carbon (IV) oxide gas is a molecular compound that does not dissociate/ionize to release H+ and thus has no effect on litmus papers.
Wet/damp/moist litmus papers contains water that dissolves/react with dry carbon (IV) oxide gas to form the weak solution of carbonic (IV) acid(H2CO3).
Carbonic (IV) acid dissociate/ionizes to a few /little free H+ and CO32-.
The few H+ (aq) ions are responsible for turning blue litmus paper to faint red showing the gas is very weakly acidic.
Chemical equation
H2CO3(aq) → 2H+(aq) + CO32-(aq) - Explain why Carbon (IV)oxide cannot be prepared from the reaction of:
- marble chips with dilute sulphuric(VI)acid.
Explanation
Reaction forms insoluble calcium sulphate(VI)that cover/coat unreacted marble chips stopping further reaction
Chemical equation
CaCO3(s) + H2SO4(aq) → CaSO4(s) + H2O(l) + CO2(g)
PbCO3(s) + H2SO4(aq) → PbSO4(s) + H2O(l) + CO2(g)
BaCO3(s) + H2SO4(aq) → BaSO4(s) + H2O(l) + CO2(g) - Lead(II)carbonate with dilute Hydrochloric acid.
Reaction forms insoluble Lead(II)Chloride that cover/coat unreacted Lead(II) carbonate stopping further reaction unless the reaction mixture is heated. Lead(II)Chloride is soluble in hot water.
Chemical equation
PbCO3(s) + 2HCl(aq) → PbCl2(s) + H2O(l) + CO2(g)
- marble chips with dilute sulphuric(VI)acid.
- Describe the test for the presence of Carbon (IV)oxide.
Using burning splint
Lower a burning splint into a gas jar suspected to contain Carbon (IV)oxide gas.The burning splint is extinguished.
Using Lime water.
Bubble the gas suspected to be Carbon (IV)oxide gas. A white precipitate that dissolve in excess bubbling is formed.
Chemical equation
Ca(OH)2(aq) + CO2(g) → CaCO3(s) + H2O(l)
CaCO3(aq) + H2O(l) + CO2(g) → Ca(HCO3)2(aq) - State three main uses of Carbon (IV)oxide gas
- In the Solvay process for the manufacture of soda ash/sodium carbonate
- In preservation of aerated drinks
- )As fire extinguisher because it does not support combustion and is denser than air.
- In manufacture of Baking powder.
Carbon(II)Oxide (CO)
i) Occurrence
Carbon(II)oxide is found is found from incomplete combustion of fuels like petrol charcoal, liquefied Petroleum Gas/LPG.
ii)School Laboratory preparation
In the school laboratory carbon(II)oxide can be prepared from dehydration of methanoic acid/Formic acid(HCOOH) or Ethan-1,2-dioic acid/Oxalic acid(HOOCCOOH) using concentrated sulphuric(VI) acid.
Heating is necessary.
METHOD 1:Preparation of Carbon (IV)Oxide from dehydration of Oxalic/ethan-1,2-dioic acid
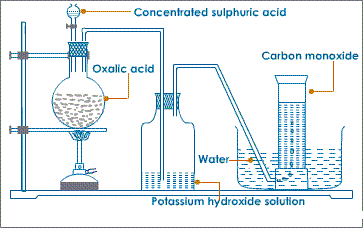
METHOD 2:Preparation of Carbon (IV)Oxide from dehydration of Formic/Methanoic acid
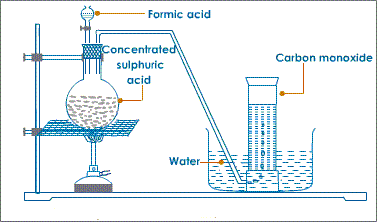
iii) Properties of Carbon (II)Oxide(Questions)
- Write the equation for the reaction for the preparation of carbon(II)oxide using;
- Method 1;
Chemical equation
HOOCCOOH(s) –Conc.H2SO4 → CO(g) + CO2(g) + H2O(l)
H2C2O4(s) –Conc.H2SO4 → CO(g) + CO2(g) + H2O(l) - Method 2;
Chemical equation
HCOOH(s) –Conc.H2SO4 → CO(g) + H2O(l)
H2CO2(s) –Conc.H2SO4 → CO(g) + H2O(l)
- Method 1;
- What method of gas collection is used during the preparation of carbon (II) oxide.
Over water because the gas is insoluble in water.
Downward delivery because the gas is 1 ½ times denser than air. - What is the purpose of:
- Potassium hydroxide/sodium hydroxide in Method 1
To absorb/ remove carbon (II) oxide produced during the reaction.
2KOH(aq) + CO2(g) → K2CO3(s) + H2O(l)
2NaOH(aq) + CO2(g) → Na2CO3(s) + H2O(l) - Concentrated sulphuric(VI)acid in Method 1 and 2.
Dehydrating agent –removes the element of water (Hydrogen and Oxygen in ratio 2:1) present in both methanoic and ethan-1,2-dioic acid.
- Potassium hydroxide/sodium hydroxide in Method 1
- Describe the smell of carbon(II)oxide.
Colourless and odourless. - State and explain the observation made when carbon(IV)oxide is bubbled in lime water for a long time.
No white precipitate is formed. - Dry and wet/moist/damp litmus papers were separately put in a gas jar containing dry carbon(IV)oxide gas. State and explain the observations made.
Observation
-blue dry litmus paper remains blue
-red dry litmus paper remains red
- wet/moist/damp blue litmus paper remains blue
- wet/moist/damp red litmus paper remains red
Explanation
Carbon(II)oxide gas is a molecular compound that does not dissociate /ionize to release H+ ions and thus has no effect on litmus papers. Carbon(II)oxide gas is therefore a neutral gas. - Carbon (II)oxide gas was ignited at the end of a generator.
- State the observations made in the flame.
Gas burns with a blue flame - Write the equation for the reaction taking place at flame K.
2CO(g) + O2(g) → 2CO2(g)
- State the observations made in the flame.
- Carbon(II)oxide is a reducing agent. Explain
Experiment
Pass carbon(II)oxide through glass tube containing copper (II)oxide. Ignite any excess poisonous carbon(II)oxide.
Observation
Colour change from black to brown. Excess carbon (II)oxide burn with a blue flame.
Explanation
Carbon is a reducing agent. It is used to reduce metal oxide ores to metal, itself oxidized to carbon(IV)oxide gas. Carbon(II)Oxide reduces black copper(II)oxide to brown copper metal
Chemical Equation
CuO(s) + CO(g) → Cu(s) + CO2(g)
PbO(s) + CO(g) → Pb(s) + CO2(g)
ZnO(s) + CO(g) → Zn(s) + CO2(g)
Fe2O3(s) + 3CO(s) → 2Fe(s) + 3CO2(g)
Fe3O4(s) + 4CO(g) → 3Fe(s) + 4CO2(g)
These reaction are used during the extraction of many metals from their ore. - Carbon (II) oxide is a pollutant. Explain.
Carbon(II)oxide is highly poisonous/toxic. It preferentially combine with haemoglobin to form stable carboxyhaemoglobin in the blood instead of oxyhaemoglobin.This reduces the free haemoglobin in the blood causing nausea, coma then death. - The diagram below show a burning charcoal stove/burner/jiko. Use it to answer the questions that follow.
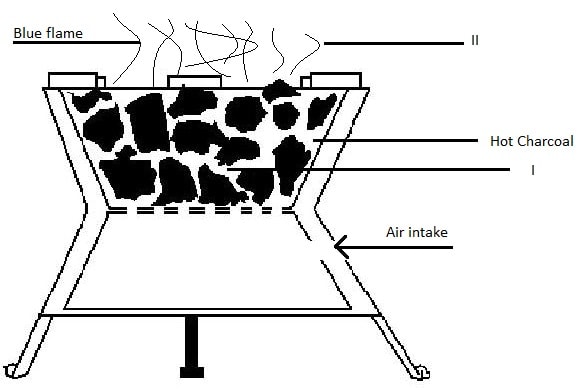
Explain the changes that take place in the burner
Explanation
Charcoal stove has air holes through which air enters. Air oxidizes carbon to carbon(IV)oxide gas at region I. This reaction is exothermic(-ΔH) producing more heat.
Chemical equation
C(s) + O2(g) → CO2(g)
Carbon(IV)oxide gas formed rises up to meet more charcoal which reduces it to Carbon(II)oxide gas.
Chemical equation
2CO2(g) + O2(g) → 2CO(g)
At the top of burner in region II, Carbon (II)oxide gas is further oxidized to Carbon(IV)oxide gas if there is plenty of air but escape if the air is limited poisoning the living things around.
Chemical equation
2CO(g) + O2(g) → 2CO2(g) - Describe the test for the presence of carbon(II)oxide gas.
Experiment
Burn/Ignite the pure sample of the gas. Pass/Bubble the products into lime water/Calcium hydroxide .
Observation
Colourless gas burns with a blue flame. A white precipitate is formed that dissolve on further bubbling of the products.
Chemical equation
2CO(g) + O2(g) → 2CO2(g) (gas burns with blue flame)
Chemical equation
Ca(OH)2(aq) + CO2(g) → CaCO3(s) + H2O(l)
Chemical equation
CO2(g) + CaCO3(s) + H2O(l) → Ca(HCO3)2(aq) - State the main uses of carbon (II)oxide gas.
- As a fuel /water gas
- As a reducing agent in the blast furnace for extracting iron from iron ore(Magnetite/Haematite)
- As a reducing agent in extraction of Zinc from Zinc ore/Zinc blende
- As a reducing agent in extraction of Lead from Lead ore/Galena
- As a reducing agent in extraction of Copper from Copper iron sulphide/Copper pyrites.
Carbonate(IV) (CO32-)and Hydrogen Carbonate(IV)(HCO3-)
- Carbonate (IV) (CO32-) are normal salts derived from carbonic(IV)acid (H2CO3) and hydrogen carbonate (IV) (HCO3-) are acid salts derived from carbonic(IV)acid. Carbonic(IV)acid(H2CO3) is formed when carbon(IV)oxide gas is bubbled in water. It is a dibasic acid with two ionizable hydrogens.
H2CO3(aq) → 2H+(aq) + CO32-(aq)
H2CO3(aq) → H+(aq) + HCO3- (aq) - Carbonate (IV) (CO32-) are insoluble in water except Na2CO3 , K2CO3 and (NH4)2CO3
- Hydrogen carbonate (IV) (HCO3-) are soluble in water. Only five hydrogen carbonates exist. NaHCO3, KHCO3, NH4HCO3, Ca(HCO3)2 and Mg(HCO3)2. Ca(HCO3)2 and Mg(HCO3)2 exist only in aqueous solutions.
- The following experiments show the effect of heat on Carbonate (IV) (CO32-) and Hydrogen carbonate (IV) (HCO3-) salts:
Experiment
In a clean dry test tube place separately about 1.0 of the following:
Zinc(II)carbonate(IV), sodium hydrogen carbonate(IV), sodium carbonate(IV), Potassium carbonate(IV) ammonium carbonate(IV), potassium hydrogen carbonate(IV), Lead(II)carbonate(IV), Iron(II)carbonate(IV), and copper(II)carbonate(IV). Heat each portion gently the strongly. Test any gases produced with lime water.
Observation- Colorless droplets form on the cooler parts of test tube in case of sodium carbonate(IV) and Potassium carbonate(IV).
- White residue/solid left in case of sodium hydrogen carbonate(IV), sodium carbonate(IV), Potassium carbonate(IV) and potassium hydrogen carbonate(IV).
- Colour changes from blue/green to black in case of copper(II)carbonate(IV).
- Colour changes from green to brown/yellow in case of Iron (II)carbonate(IV).
- Colour changes from white when cool to yellow when hot in case of Zinc (II) carbonate(IV).
- Colour changes from yellow when cool to brown when hot in case of Lead (II) carbonate(IV).
- Colourless gas produced that forms a white precipitate with lime water in all cases.
Sodium carbonate(IV) and Potassium carbonate(IV) exist as hydrated salts with 10 molecules of water of crystallization that condenses and collects on cooler parts of test tube as a colourless liquid.
Chemical equation
Na2CO3.10H2O(s) → Na2CO3(s) + 10H2O(l)
K2CO3.10H2O(s) → K2CO3(s) + 10H2O(l) - Carbonate (IV) (CO32-) and Hydrogen carbonate (IV) (HCO3-) salts decompose on heating except Sodium carbonate(IV) and Potassium carbonate(IV).
- Sodium hydrogen carbonate(IV) and Potassium hydrogen carbonate(IV) decompose on heating to form sodium carbonate(IV) and Potassium carbonate(IV). Water and carbon(IV)oxide gas are also produced.
Chemical equation
2NaHCO3(s) → Na2CO3(s) + H2O(l) + CO2(g)
2KHCO3(s) → K2CO3(s) + H2O(l) + CO2(g) - Calcium hydrogen carbonate(IV) and Magnesium hydrogen carbonate(IV) decompose on heating to form insoluble Calcium carbonate(IV) and Magnesium carbonate(IV). Water and carbon(IV)oxide gas are also produced.
Chemical equation
Ca(HCO3)2(aq) → CaCO3(s) + H2O(l) + CO2(g)
Mg(HCO3)2(aq) → MgCO3(s) + H2O(l) + CO2(g) - Ammonium hydrogen carbonate(IV) decompose on heating to form ammonium carbonate(IV). Water and carbon(IV)oxide gas are also produced.
Chemical equation
2NH4HCO3(s) → (NH4)2CO3(s) + H2O(l) + CO2(g) - All other carbonates decompose on heating to form the metal oxide and produce carbon(IV)oxide gas e.g.
Chemical equation
MgCO3(s) → MgO(s) + CO2(g)
BaCO3(s) → BaO(s) + CO2(g)
CaCO3(s) → CaO(s) + CO2(g)
CuCO3(s) → CuO(s) + CO2(g)
ZnCO3(s) → ZnO(s) + CO2(g)
PbCO3(s) → PbO(s) + CO2(g)
- Sodium hydrogen carbonate(IV) and Potassium hydrogen carbonate(IV) decompose on heating to form sodium carbonate(IV) and Potassium carbonate(IV). Water and carbon(IV)oxide gas are also produced.
- The following experiments show the presence of Carbonate (IV) (CO32-) and Hydrogen carbonate (IV) (HCO3-) ions in sample of a salt:
- Using Lead(II) nitrate(V)
I. Using a portion of salt solution in a test tube, add four drops of Lead(II)nitrate(V)solution. Preserve. Observation inference White precipitate/ppt CO32-, SO32- ,SO42-,Cl-
-II. To the preserved solution, add six drops of dilute nitric(V)acid. Preserve. Observation inference White precipitate/ppt persists White precipitate/ppt dissolves
SO42- ,Cl-
CO32- ,SO32-
II. To the preserved sample( that forms a precipitate ),heat to boil. Observation inference White precipitate/ppt persists
White precipitate/ppt dissolvesSO42-
Cl-III. To the preserved sample( that do not form a precipitate ),add three drops of acidified potassium manganate(VII)/lime water Observation inference Effervescence/bubbles/fizzing colourless gas produced Acidified KMnO4 decolorized/no white precipitate on lime water Effervescence/bubbles/fizzing colourless gas produced Acidified KMnO4 not decolorized/white precipitate on lime water
SO32-
CO32-
- Using Barium(II)nitrate(V)/ Barium(II)chloride
ExplanationsI. To about 5cm3 of a salt solution in a test tube add four drops of Barium(II) nitrate (V) / Barium(II)chloride. Preserve.
Observation Inference White precipitate/ppt SO42-, SO32-, CO32- ions
II. To the preserved sample in (I) above, add six drops of 2M nitric(V) acid. Preserve.
Observation Inference White precipitate/ppt persists
White precipitate/ppt dissolves
SO42- ions
SO32-, CO32-, ions
III.To the preserved sample observation 2 in (II) above, add 4 drops of acidified potassium manganate(VII) /dichromate(VI).
Observation Inference (i)acidified potassium manganate(VII)decolorized
(ii)Orange colour of acidified potassium dichromate(VI) turns to green(i)acidified potassium manganate(VII) not decolorized
(ii)Orange colour of acidified potassium dichromate(VI) does not turns to greenSO32- ions
CO32- ions
- Using Lead(II)nitrate(V)
- Lead(II)nitrate(V) solution reacts with chlorides(Cl-), Sulphate (VI) salts (SO42-), Sulphate (IV)salts (SO32-) and carbonates(CO32-) to form the insoluble white precipitate of Lead(II)chloride, Lead(II)sulphate(VI), Lead(II) sulphate (IV) and Lead(II)carbonate(IV).
Chemical/ionic equation:
Pb2+(aq) + Cl-(aq) → PbCl2(s)
Pb2+(aq) + SO42+(aq) → PbSO4(s)
Pb2+(aq) + SO32+(aq) → PbSO3(s)
Pb2+(aq) + CO32+(aq) → PbCO3(s) - When the insoluble precipitates are acidified with nitric(V) acid,
- Lead(II)chloride and Lead(II)sulphate(VI) do not react with the acid and thus their white precipitates remain/ persists.
- Lead(II) sulphate (IV) and Lead(II)carbonate(IV) reacts with the acid to form soluble Lead(II) nitrate (V) and produce/effervesces/fizzes/bubbles out sulphur(IV)oxide and carbon(IV)oxide gases respectively.
Chemical/ionic equation:
PbSO3(s) + 2H+(aq) → H2O(l) + Pb2+(aq) + SO2(g)
PbCO3(s) + 2H+(aq) → H2O(l) + Pb2+(aq) + CO2(g)
- When Lead(II)chloride and Lead(II)sulphate(VI) are heated/warmed;
- Lead(II)chloride dissolves in hot water/on boiling(recrystallizes on cooling)
- Lead(II)sulphate(VI) do not dissolve in hot water thus its white precipitate persists/remains on heating/boiling.
- When sulphur(IV)oxide and carbon(IV)oxide gases are produced;
- sulphur(IV)oxide will decolorize acidified potassium manganate(VII) and/or Orange colour of acidified potassium dichromate(VI) will turns to green.
- Carbon(IV)oxide will not.
Chemical equation:
5SO32-(aq) + 2MnO4-(aq) +6H+(aq) → 5SO42-(aq) + 2Mn2+(aq) + 3H2O(l)
3SO32-(aq) + Cr2O72-(aq) +8H+(aq) → 3SO42-(aq) + 2Cr3+(aq) + 4H2O(l) - Carbon(IV)oxide forms an insoluble white precipitate of calcium carbonate if three drops of lime water are added into the reaction test tube when effervescence is taking place. Sulphur(IV)oxide will not.
Chemical equation:
Ca(OH)2(aq) + CO2(g) → CaCO3(s) + H2O(l)
These tests should be done immediately after acidifying to ensure the gases produced react with the oxidizing agents/lime water.
- Lead(II)nitrate(V) solution reacts with chlorides(Cl-), Sulphate (VI) salts (SO42-), Sulphate (IV)salts (SO32-) and carbonates(CO32-) to form the insoluble white precipitate of Lead(II)chloride, Lead(II)sulphate(VI), Lead(II) sulphate (IV) and Lead(II)carbonate(IV).
- Using Barium(II)nitrate(V)/ Barium(II)Chloride
- Barium(II)nitrate(V) and/ or Barium(II)chloride solution reacts with Sulphate (VI) salts (SO42-), Sulphate (IV)salts (SO32-) and carbonates(CO32-) to form the insoluble white precipitate of Barium(II)sulphate(VI), Barium(II) sulphate (IV) and Barium(II)carbonate(IV).
Chemical/ionic equation:
Ba2+(aq) + SO42+(aq) → BaSO4(s)
Ba2+(aq) + SO32+(aq) → BaSO3(s)
Ba2+(aq) + CO32+(aq) → BaCO3(s) - When the insoluble precipitates are acidified with nitric(V) acid,
- Barium (II)sulphate(VI) do not react with the acid and thus its white precipitates remain/ persists.
- Barium(II) sulphate (IV) and Barium(II)carbonate(IV) reacts with the acid to form soluble Barium(II) nitrate (V) and produce /effervesces /fizzes/ bubbles out sulphur(IV)oxide and carbon(IV)oxide gases respectively.
Chemical/ionic equation:
BaSO3(s) + 2H+(aq) → H2O(l) + Ba2+(aq) + SO2(g)
BaCO3(s) + 2H+(aq) → H2O(l) + Ba2+(aq) + CO2(g)
- When sulphur(IV)oxide and carbon(IV)oxide gases are produced;
- sulphur(IV)oxide will decolorize acidified potassium manganate(VII) and/or Orange colour of acidified potassium dichromate(VI) will turns to green. Carbon(IV)oxide will not.
Chemical equation:
5SO32-(aq) + 2MnO4-(aq) +6H+(aq) → 5SO42-(aq) + 2Mn2+(aq) + 3H2O(l)
3SO32-(aq) + Cr2O72-(aq) +8H+(aq) → 3SO42-(aq) + 2Cr3+(aq) + 4H2O(l) - Carbon(IV)oxide forms an insoluble white precipitate of calcium carbonate if three drops of lime water are added into the reaction test tube when effervescence is taking place. Sulphur(IV)oxide will not.
Chemical equation:
Ca(OH)2(aq) + CO2(g) → CaCO3(s) + H2O(l)
These tests should be done immediately after acidifying to ensure the gases produced react with the oxidizing agents/lime water.
- sulphur(IV)oxide will decolorize acidified potassium manganate(VII) and/or Orange colour of acidified potassium dichromate(VI) will turns to green. Carbon(IV)oxide will not.
- Barium(II)nitrate(V) and/ or Barium(II)chloride solution reacts with Sulphate (VI) salts (SO42-), Sulphate (IV)salts (SO32-) and carbonates(CO32-) to form the insoluble white precipitate of Barium(II)sulphate(VI), Barium(II) sulphate (IV) and Barium(II)carbonate(IV).
- Using Lead(II) nitrate(V)
Sodium Carbonate(IV) (Na2CO3)
(a) Extraction of Sodium Carbonate from Soda Ash
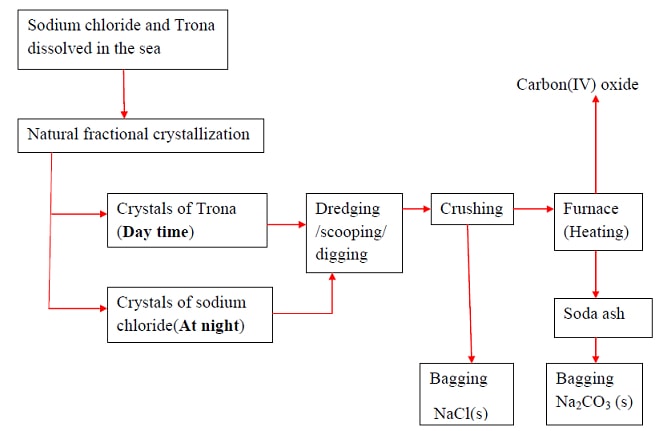
- Sodium carbonate naturally occurs in Lake Magadi in Kenya as Trona.
- Trona is the double salt ; sodium sesquicarbonate. NaHCO3.Na2CO3.H2O.
- It is formed from the volcanic activity that takes place in Lake Naivasha, Nakuru ,Bogoria and Elementeita .All these lakes drain into Lake Magadi through underground rivers. Lake Magadi has no outlet.
- Solubility of Trona decrease with increase in temperature.
- High temperature during the day causes trona to naturally crystallize. It is mechanically scooped/dredged/dug and put in a furnace.
- Inside the furnace, trona decompose into soda ash/sodium carbonate.
Chemical equation
2NaHCO3.Na2CO3.H2O(s) → 3Na2CO3(s) + 5H2O(l) + CO2(g) - Soda ash is then bagged and sold as Magadi soda.It is mainly used:
- in making glass to lower the melting point of raw materials (sand/SiO2 from 1650oC and CaO from 2500oC to around 1500oC)
- in softening hard water
- in the manufacture of soapless detergents.
- Swimming pool “pH increaser”
- Sodium chloride is also found dissolved in the lake.
- Solubility of sodium chloride decrease with decreases in temperature/ sodium chloride has lower solubility at lower temperatures.
- When temperatures decrease at night it crystallize out.
- The crystals are then mechanically dug/dredged /scooped then packed for sale as animal/cattle feeds and seasoning food.
Summary Flow Diagram Showing the Extraction of Soda Ash from Trona
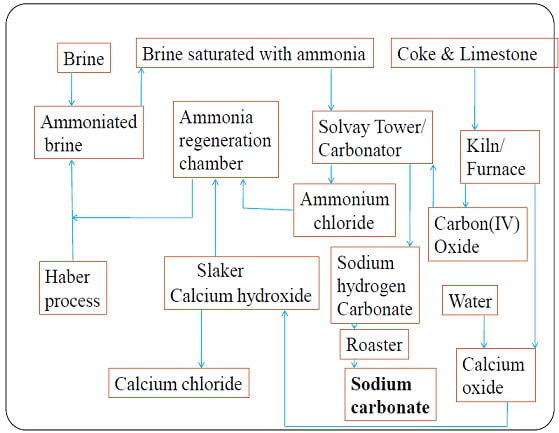
b)The Solvay Process for Industrial Manufacture of Sodium Carbonate(IV)
(i) Raw materials.
- Brine /Concentrated Sodium chloride from salty seas/lakes.
- Ammonia gas from Haber.
- Limestone /Calcium carbonate from chalk /limestone rich rocks.
- Water from rivers/lakes.
(ii) Chemical processes
- Ammonia gas is passed up to meet a downward flow of sodium chloride solution/brine to form ammoniated brine/ammoniacal brine mixture in the ammoniated brine chamber
- The ammoniated brine mixture is then pumped up, atop the carbonator/ solvay tower.
- In the carbonator/ solvay tower, ammoniated brine/ammoniacal brine mixture slowly trickle down to meet an upward flow of carbon(IV)oxide gas.
- The carbonator is shelved /packed with quartz/broken glass to
- reduce the rate of flow of ammoniated brine/ammoniacal brine mixture.
- increase surface area of the liquid mixture to ensure a lot of ammoniated brine/ammoniacal brine mixture react with carbon(IV)oxide gas.
- Insoluble sodium hydrogen carbonate and soluble ammonium chloride are formed from the reaction.
Chemical equation
CO2(g) + H2O(l) + NaCl(aq) + NH3(g) → NaHCO3(s) + NH4Cl(aq) - The products are then filtered. Insoluble sodium hydrogen carbonate forms the residue while soluble ammonium chloride forms the filtrate.
- Sodium hydrogen carbonate itself can be used:
- as baking powder and preservation of some soft drinks.
- as a buffer agent and antacid in animal feeds to improve fibre digestion.
- making dry chemical fire extinguishers.
- In the Solvay process Sodium hydrogen carbonate is then heated to form Sodium carbonate/soda ash, water and carbon (IV) oxide gas.
Chemical equation
2NaHCO3(s) → Na2CO3(s) + CO2(g) + H2O(l) - Sodium carbonate is stored ready for use in:
- during making glass/lowering the melting point of mixture of sand/SiO2 from 1650oC and CaO from 2500oC to around 1500oC
- in softening hard water
- in the manufacture of soapless detergents.
- swimming pool “pH increaser”.
- Water and carbon(IV)oxide gas are recycled back to the ammoniated brine/ammoniacal brine chamber.
- More carbon(IV)oxide is produced in the kiln/furnace. Limestone is heated to decompose into Calcium oxide and carbon(IV)oxide.
Chemical equation
CaCO3(s) → CaO(s) + CO2(g) - Carbon(IV)oxide is recycled to the carbonator/solvay tower. Carbon (IV)oxide is added water in the slaker to form Calcium hydroxide. This process is called slaking.
Chemical equation
CaO(s) + H2O(l) → Ca(OH)2(aq) - Calcium hydroxide is mixed with ammonium chloride from the carbonator/solvay tower in the ammonia regeneration chamber to form Calcium chloride, water and more ammonia gas.
Chemical equation
Ca(OH)2(aq) +2NH4Cl(aq) → CaCl2(s) + 2NH3(g) + H2O(l) - NH3(g) and H2O(l) are recycled.
- Calcium chloride may be used:
- as drying agent in the school laboratory during gas preparation (except ammonia gas)
- to lower the melting point of solid sodium chloride / rock salt salts during the Downs process for industrial extraction of sodium metal.
Detailed Summary Flow Diagram of Solvay Process
Download CARBON AND IT'S COMPOUNDS - Chemistry Notes Form 2.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students