Questions
- In an experiment magnesium ribbon was heated in air. The product formed was found to be heavier than the original ribbon. Potassium manganate (VII) was on the other hand, heated in air and product formed was found to be lighter. Explain the differences on the observation made
- In a filtration experiment 25cm3 of a solution of Sodium Hydroxide containing 8g per litre was required for complete neutralization of 0.245g of a dibasic acid. Calculate the relative molecular mass of the acid (Na = 23.0, O = 16, H= 1)
- D grams of Potassium hydroxide were dissolved is distilled water to make 100 cm3 of solution. 50cm3 of the solution required 50cm3 of 2.0M nitric acid for complete neutralization.
Calculate the mass D of Potassium hydroxide (RFM of KOH = 56)
KOH(aq) + HNO3(aq) → KNO3(aq) + H2O(l) - When excess dilute hydrochloric acid was added to sodium sulphite, 960cm3 of sulphuric (IV) Oxide gas was produced. Calculate the mass of sodium sulphate that was used.
(Molar gas volume = 24000cm3 and Molar mass of sulphite = 126g) - The equation of the formation of iron (III) chloride is
2Fe(s) + 3Cl2(g) → 2FeCl3
Calculate the volume of chlorine which will react with iron to form 0.5g of Iron (III) chloride.
(Fe = 56 Cl=35.5). Molar gas volume at 298K = 24dm3) - 15.0cm3 of ethanoic acid (CH3COOH) was dissolved in water to make 500cm3 of solution. Calculate the concentration of the solution in moles per litre [C=12, H = 1, O = 16, density of ethanoic acid is 1.05g/cm3]
- When 1.675g of hydrated sodium carbonate was reacted with excess hydrochloric acid, the volume carbon (IV) oxide gas obtained at room temperature and pressure was 150cm3. Calculate the number of moles of water of crystallization in one mole of hydrated sodium carbonate:- (Na=23, H =1, C=12, O=16, MGV at R.T.P = 24000cm3)
- How many chloride ions are present in 1.7g of magnesium chloride crystals? (Avogadro’s constant = 6.0 x 1023, Mg = 24, Cl = 35.5)
- Calculate the volume of oxygen gas used during the burning of magnesium (O = 16, molar gas volume = 24,000cm3 at room temperature)
- A hydrated salt has the following composition by mass. Iron 20.2 %, oxygen 23.0%, sulphur 11.5%, water 45.3%
- Determine the formula of the hydrated salt (Fe=56, S=32, O=16, H=11)
- 6.95g of the hydrated salt in (i) above were dissolved in distilled water and the total volume made to 250cm3 of solution. Calculate the concentration of the resulting salt solution in moles per litre. (Given that the molecula mass of the salt is 278)
-
- Lead (II) ions react with iodide ions according to the equation;
Pb2+(aq) + 2I−(aq) → PbI2(s)
300cm3 of a 0.1m solution of iodide ions was added to a solution containing excess lead (II) ions. Calculate the mass in grams of lead II iodide formed - Identify the colour of the product formed in (i)
- Lead (II) ions react with iodide ions according to the equation;
-
- The diagram below represents part of the structure of sodium chloride crystal
The position of one of the sodium ions in the crystal is shown as;- On the diagram, mark the positions of the other three sodium ions
- The melting and boiling points of sodium chloride are 801oC and 1413oC respectively. Explain why sodium chloride does not conduct electricity at 25oC, but does not at temperatures between 801oC and 1413oC
- Give a reason why ammonia gas is highly soluble in water
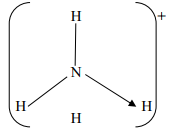
- The structure of ammonium ion is shown below;
Name the type of bond represented in the diagram by N → H - Carbon exists in different crystalline forms. Some of these forms were recently discovered in soot and are called fullerenes
- What name is given to different crystalline forms of the same element
- Fullerenes dissolve in methylbenzene while the other forms of carbon do not. Given that soot is a mixture of fullerenes and other solid forms of carbon, describe how crystals of fullerenes can be obtained from soot
- The relative molecular mass of one of the fullerenes is 720. What is the molecular mass of this fullerene
- The diagram below represents part of the structure of sodium chloride crystal
- Calculate the volume of oxygen gas used during the burning of magnesium (O = 16, molar gas volume = 24,000cm3 at room temperature)
- Study the information in the table below and answer the questions that follow
Number of carbon atoms per molecule Relative molecular mass of the hydrocarbon 2 28 3 42 4 56 - Write the general formula of the hydrocarbons in the table
- Predict the relative atomic mass of the hydrocarbons with 5 carbon atoms
- Determine the relative atomic mass of the hydrocarbon in (ii) above and draw its structural formula (H=1.0, C=12.0)
- Galvanized iron sheets are made by dipping the sheets in molten Zinc.
- Explain how zinc protects iron from rusting
- Name the process applied in galvanization of iron with zinc
- A factory uses nitric acid and ammonia gas as the only reactant for the preparation of the fertilizer if the daily production of the fertilizer is 4800kg. Calculate the mass of ammonia gas used daily (N = 14.0, O= 16.0, H = 1.0)
- Calculate the volume of sulphur (VI) oxide gas that would be required to produce 178kg of oleum in step 3 molar gas volume at s.t.p = 22.4 litres (H = 1, O = 16, S = 32)
- A sample of biogas contains 35.2% by mass of methane. A biogas cylinder contains 5.0kg of the gas. Calculate:
- Number of moles of methane in the cylinder (Molar mass of methane = 16)
- Total volume of carbon (IV) oxide produced by the combustion of methane in the cylinder (Molar gas volume = 24.0dm3 at room temperature and pressure)
- 0.84g of aluminium were reacted completely with chlorine gas. Calculate the volume of chlorine gas used. (Molar gas volume is 24dm3, Al = 27)
- 3.52g of Carbon (IV) Oxide and 1.40g of water are produced when a mass of a hydrocarbon is completely burnt in oxygen. Determine the empirical formula of the hydrocarbon; (H = 1 , C= 12, O = 16)
- Calculate the number of water molecules when 34.8g Na2CO3.xH2O is heated and 15.9g of anhydrous Na2CO3 obtained (H=1, O=16, Na= 23, C = 12)
- A weighed sample of crystallined sodium carbonate (Na2CO3.nH2O) was heated in a crucible until there was no further change in mass. The mass of the sample reduced by 14.5%. Calculate the number of moles (n) of water of crystallization (Na = 23, O = 16, C = 12, H = 1)
- In a reaction 20cm3 of 0.1 M Sodium Carbonate completely reacted with 13cm3 of dilute sulphuric acid. Find the molarity of the sulphuric acid used.
- An organic compound P contains 68.9% carbon, 13.5% hydrogen and 21.6% oxygen. The relative formula mass of p is 74. Determine its molecular formula. [C=12, H=1, O=16]
- Campers GAZ cylinder contains about 1.12dm3 of butane measured at 0o and 1atm. Given that 25% of heat is lost, what is the maximum volume of water at room temperature which can be boiled to 100oC in order to make some coffee?
C4H10(g) + 6½O2(g) → 4CO2(g) + 5H2O(l); ΔHθ = -3,000KJmol-1
(Specific heat capacity of water = 4.2J g-1oC, density of water 1gcm-3 Molar gas volume 22.41dm3 at s.t.p) - An aqueous solution containing anhydrous sodium carbonate was prepared by dissolving 19.6g of the salt in 250cm3 of distilled. Calculate the volume of 2M of magnesium chloride solution required to precipitate all the carbonate ions in the solution. (Na=23, C= 12; O = 16; Mg = 24; Cl =35.5)
- 10.08g of ethanedioic acid (H2C2O4.xH2O) crystals were dissolved in water and made to 1dm3 solution. 25.0 cm3 of this solution was completely neutralized by 20cm3 of 0.2M sodium hydroxide solution.
Calculate- Molarity of the acid
- the value of x in H2C2O4.xH2O acid
- 1.6g of magnesium metal is reacted with excess hydrochloric acid. Calculate the volume of hydrogen gas produced (Molar gas volume at stp = 22.4dm3 Mg=24)
- 60 litres of sulphur(IV) oxide were made to react with 40 litres of oxygen.
- Which reactant was in excess and by how much?
- What is the volume of the product?
- During welding of cracked railway lines by thermite 12.0g of oxide of iron is reduced by aluminium to 8.40g of iron. Determine the empirical formula of the oxide (Fe= 56.0, O= 16.0)
- 0.84g of aluminium reacted completely with chlorine gas. Calculate the volume of chlorine gas used (Molar gas volume is 24dm3, Al = 27)
Answers
- When a magnesium ribbon is heated in air it combines with oxygen forming magnesium oxide.
When potassium manganate (VII) is heated it decomposes giving off oxygen which escapes in air - RFM of NaOH = 40
Moles of NaOH = 8/40 = 0.2M ✓
Moles of NaOH in 25cm3
25 x 0.2 = 0.005
1000
Mole ratio 1:2
Moles of acid = 0.005/2
= 0.0025
1 x 0.245 = 98
0.0025 - No. Of moles of HNO3 acid
50 x 2 = 0.1moles
1000
Mole ratio 1:1
The KOH will have 0.1moles; 0.1 × 100 = 0.2moles
50
Then D grams is 0.2 × 56
= 11.2g - Number of moles of Q = 960 cm3 x 1 mole
24000 cm3
= 0.04moles
Equation:
Na2SO3(s) + 2HCl(aq) → 2NaCl(aq) + SO2(g) + H2O(l)
Mole ratio Na2SO3 : SO2 is 1:1
∴No. of moles of Na2SO3 = 0.04moles
Mass of Na2SO3 = 126gmol-1 x 0.04
= 5.04g - From the equation
- (3x24) litres of chlorine react with iron to produce [(56 x 2) + (35.5 X3)] g of FeCl3.
325 g of Fecl3 is produced by 72 litres of Cl2
Then 0.5g of Fecl3 is produced by:
0.5 x 72 =0.11078 litres
325
= 110.78 cm3 - RMM (CH3OOH) = 60
Mass of 15cm3 and = 1.05 x 15 = 15.75g
Moles in 500cm3 solution = 15.75 = 0.2625
60
Molarity = 1000 x 0.2625
5000
= 0.525M - If 24000 cm3 = 1mole
150cm3 = ?
150 x 1
24000
= 0.00625 moles of CO2
Since the ratio of Na2CO3; O2 produced is 1:1 the mass of Na2CO3 = 0.00625 x 106 = 0.6625g
Na2CO3 H2O Mass 0.6625g
RFM 106
Mole 0.6625/106 = 0.00625
Ratio 0.00625/0.00625
= 1
Na2CO3.9H2O1.0125g
18
1.0125/18 = 0.5625
0.05625/0.00625
= 9 - MgCl2 → Mg2+(s) + 2Cl
R.F.M of MgCl2 = 24 + 71
= 95
Moles of Mass = 1.7
R.F.M 95
= 0.01789moles
1 mole of MgCl2 = 2moles of Cl- ions
0.01789moles of MgCl2 = 0.01789 x 2
= 0.03478 moles of Cl- ions
1mole = 6.0 x 1023 ions
0.03578moles = 0.03578 x 6.0x 1023
1
= 2.1468 x 1022 ions of Cl- - Mass of O2 = (4.0 – 2.4)= 1.6g
Moles of O2 = 1.6/16 = 0.1
If 1 mol O2 ________ 24000cm3
0.1 Mol Mg = 0.5 mol O2 = 1200cm3
OR
2mg : O2
2(24) 24000
2.4/2(24) = x/240000
x = 2.4 x 24000 = 1200cm3
2(2.4) -
- Fe S O H2O
20.2/56 11.5/32 23.0/16 45.3/18
0.36/0.36 0.36/0.36 1.44/0.36 2.52/0.36
1 1 4 7
Empirical formula: FeSO4.7H2O - 6.95g = 6.95/278 = 0.025
∴ 0.05 moles in 250cm3 = 0.025 x 1000/250 = 0.1
- Fe S O H2O
-
- R.F.M of PbI2 = 207 + (127 × 2) = 461
2 moles of I- ions produces 1 mole of PbI2
Moles of I-ions = 0.1 × 300 = 0.03 mole
1000
Mole ratio PbI2 : I- mole of PbI2 formed = 0.03/2 = 0.05
1 : 2
Mass of PbI2 formed = 0.015 mole × 461
= 6.915 g - Yellow precipitateMocks Topical Analysis www.easyelimu.com 20
- R.F.M of PbI2 = 207 + (127 × 2) = 461
-
-
- At 25o C, sodium chloride is in solid form. Ions cannot move. Between 801oC and 1413oC sodium chloride is in liquid state, ions are mobile
- Both ammonia and water are polar moleculer and hydrogen bonds are formed
- N → H // co-ordinate bond / Dative bond
-
- Allotrope
- Add methylbenzene to soot in a beaker. Shake and filter. Warm the filtrate to concentrate it. Allow the concentrate to cool for crystals to form. Filter to obtain crystals of fullerene
- 720/12 = 60
-
- Mass of O2 = (4.0 – 2.4)= 1.6g
Moles of O2 = 1.6/16 = 0.1
If 1 mol O2 → 24000cm3
0.1 Mol Mg = 0.5 mol O2 = 1200cm3
OR
2mg : O2
2(24) 24000
2.4/2(24) = x/240000
x = 2.4 x 24000 = 1200cm3
2(2.4) -
- CnH2n, where n = No. of carbon atoms
- 70
- C5H10, CH3CH=CHCH2CH3
OR CH3CH2CHCH2 = CH2
-
- Zinc is more reactive// higher reduction potential than copper it will react with// get oxidized in preference to iron oxygen to form Zinc Oxide coat which protects iron from rusting
- Sacrificial protection or cathodic protection
- NH3(g) + HNO3(g) → NH4NO3(s)
RMM of NH4NO3 = 80
Moles of NH4NO3 = 4800/80 = 60moles
RMM of NH3 = 17
Mass of NH3 = 60 x 17 = 1020KJ - From the equation of step 3
SO3(g) + H2SO4(l) → H2S2O7(l)
RFM of H2S2O7 = 2 + (2 × 32) + (7 × 16) = 178 ✓ ½ mark
178g of Oleum are produced by 22.4 liters of SO3 ✓ ½ mark
178 kg of Oleum will produce
178 × 1000 × 22.4l ✓ 1½ mark
178g
= 22,4000 liters ✓ ½ mark
(Total 13 marks) -
- 35.2 x 1000
100 x 16
= 10Moles
Or mass of CH4 = 35.2 x 5 = 1.76g
1000
Mass in g = 1.76 x 1000 = 1760kg
Moles of methane = 1760/16
= 110 Moles - CH4 + 2O2 → CO2 + 2H2O – (ignore states)
Volume = 110 x 24.0
= 2640 dm3
Mark consequential from equation and b(ii) (Without equation max *TZM*)
- 35.2 x 1000
- Volume of Cl2 used
= 0.047 x 24
= 1.128dm3 - Mass due Carbon in CO2 = 12/4 x 35.2
= 0.96
Moles carbon = 0.96/12 = 0.08
Mass due Hydrogen in H2O = 2/18 x 1.40
= 0.156
Moles hydrogen = 0.156/1 = 0.156
Mole ratio C:H = 1:1.95
E.F = CH2 - Na2CO3.xH2O → Na2CO3 + H2O √1
34.8g 15.9g 18.9g
106 18
0.15 √1 1.15
0.15 0.15
x = 7 √1
Total 3 marks - % of H2O lost = 14.5%^
5 of anhydrous Na2CO3 = 85.5% (½mk)
R.F.M of Na2CO3 = 106 (½mk)
RMM of H2O = 18 (½mk)
n = 1 (Na2CO3.H2O) (½mk)Na2CO3 H2O 85.5
106
0.8066
0.805514.5
18
0.8066
0.8055 - Moles of Na2CO3 = 20 x 0.1 = 0.002 moles
1000
Na2CO3 + H2SO4(aq) → Na2SO4(aq) + H2O(l) + CO2(g)
Mole ratio 1 : 1
Moles of H2SO4 = Moles of Na2CO3
= 0.002 moles
Molarity of H2SO4 = 10000 x 0.002 = 0.154 moles
13 -
Element C H O % 68.9 13.5 21.6 Molar mass 12 1 16 Moles 68.9/12
5.40313.5/1
13.5216/16
1.35MR 5.403/1.33
413.5/1.35
101.35/1.35
1Ratio 4 10 1
h (C4H10O) = 74
h (12 x 4) + (10 x 1) +16 = 74
74h = 74
h= 1
Formula C4H10O - Moles C4H10 = 1.12/22.4 = 0.05 mol
Heat produced + 0.05 × (3000) = 150 kJ
Usefull heat = 75×150 = 112.5 kJ
100
Let volume of water = V
Room temperature = 25oC
Boiling point = 100oC
Change in temperature, ΔT = 100 − 25 = 75oC ½ mk
Q = ΔT × mass × C
= 75 × V × 4.2 =112500
315V = 112500
V = 112500/315 ½ mk
V = 357cm3 ½ mk - RFM Na2CO3 = 43 + 12 + 48 = 106
Mol. Na2CO3 = 19.6/106 = 0.8149057
Molarity of Na2CO3 = 0.1849057/0.25 = 0.73962M
Na2CO3(aq) + MgCl2(aq) → MgCO3(s) + 2NaCl(aq)
Mole ratio NaCO3 : MgCl2 is 1:1
∴ mol. MgCl2 Reacted = 0.1849
If 2.0 mol. = 1000cm3 solution MgCl2
= 0.1849mol = 0.1849 × 1000
2
= 92.45 or 92.5 cm3 -
- ACID BASE
1 2
½ ×0.004 20cm3 × 0.2 moles
= 0.002 moles ✓½ 1000cm3 = 0.004 moles
25cm3 ________ 0.002 moles ✓ ½
1000cm3 ______ ?
1000cm3 × 0.002 moles = 0.08M✓ ½ - 0.08 moles ________________ 10.08g H2C2O4.xH2O ✓ ½
1 mole __________________ ?
1 mole × 10.08 = 126 ✓ ½
0.08 moles
126 _______ H2C2O4.xH2O
18x = 126 – 90 ✓ ½
18x = 36
x = 2 ✓ ½
- ACID BASE
- Mg(g) + 2HCl (aq) → MgCl2 (aq) + H2 (g)
24g ___________22.4dm3
16g __________?
1.6g x 22.4dm3 ✓ ½ = 1.4933 dm3
24g -
- 2SO2(g) + O2(g) → 2SO3(g), SO2 : O2
2 1 2 60 : 30 ✓½
60l 40l
Oxygen ✓½ by 10 litres - 60 litres
- 2SO2(g) + O2(g) → 2SO3(g), SO2 : O2
- Mass of Oxygen = 12 – 8.4 = 3.5g
Element Fe O Mass 8.4 3.6 RAM 56 16 No. of moles 8.4/56
0.153.6/16
0.225Mole ration 0.15/0.15
1
20.225/0.15
1.5 × 2
3
The empirical formula is Fe2O3 -
2AL 3CL2 = 2ALCL3
Mole of alumminium 0.84 = 0.0311.bt accrdin 2 d equation 2 mole of AL react with 3mole of Cl.
27
1mole of Al required 1.5mole of cl
0.0311mole of Al required 0.0311 ×1.5mole of Cl = 0.04665mole.
1 mole of a gas occupied 22.4dm3,0.04665 mole will occupy 0.04665 × 22.4 = 1.0450dm3 of Cl
Download The Mole Questions and Answers - Chemistry Form 3 Topical Revision.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students