Get these free chemistry form 1 topical revision questions and answers for your exam preparation at national and local levels. These chemistry topical revision questions are compiled from the numerous high school KICD authorized textbooks including, KLB chemistry form 1, Finder chemistry textbook form 1, Mentors chemistry form 1, Spotlight chemistry form 1, Moran chemistry form 1, Pearsons chemistry form 1, etc. The topics covered by the topical revision questions for chemistry are shown below.
With the aid of these topical revision questions, you can review the following form 1 chemistry concepts:
You can get the topical revision questions for free without having to pay a cent on our EasyElimu Study App, which you can get from the Google Playstore here.
As a bonus, if you need to brush up your knowledge on a topic, EasyElimu has summarized notes, so you won’t have to stress over writing that MwaKenya, which makes passing that summative chemistry form 1 term 3 exam easy. Whether it’s term 1, 2 or 3, these free chemistry form 1 topical revision questions can help you get an A
Easy Elimu is a chemistry revision questions and answers app. Though it contains all subjects not only chemistry.
For free access to the answers to the topical revision questions, visit the EasyElimu App.
Alternatively, you can download the individual topical revision questions and answers in PDF format from this page by clicking the blue download notes icon under each topic.
KCSE Chemistry Revision Questions and Answers PDF
 |
 |
 |
 |
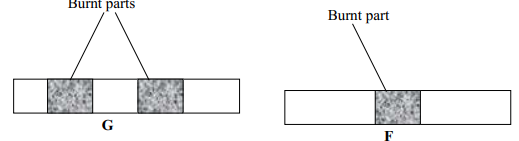
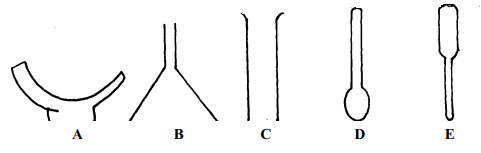
| Diagram | Name | Use |
 |
(½mk ) | (½mk) |
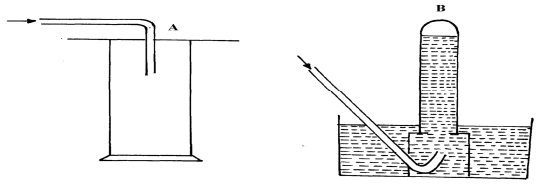
 |
(½mk) | (½mk) |
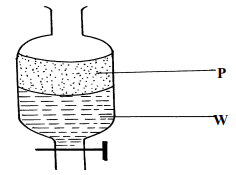
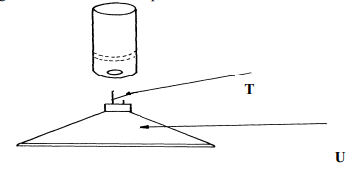 |
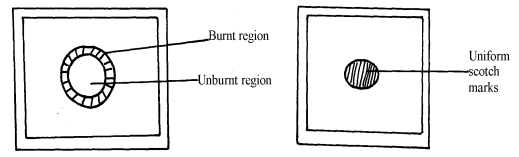 |
| Ions | Electron arrangement | Ionic radius (nm) |
| A+ B+ C2+ |
2.8 2.8.8 2.8 |
0.95 0.133 0.065 |
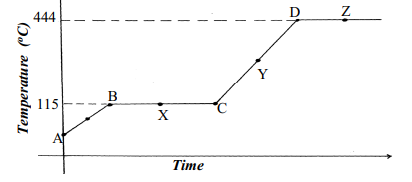 |
| Solvent | Number of spots |
| X | 6 |
| Y | 2 |
| Z | 3 |
| ELEMENTS PHYSICAL STATE AT ROOM TEMPERATURE | P SOLID | R SOLID |
| Physical states of products | White solid powder only | Colourless gases L and M |
| Nature of solutions in water | Basic | L strongly acidic M slightly acidic |
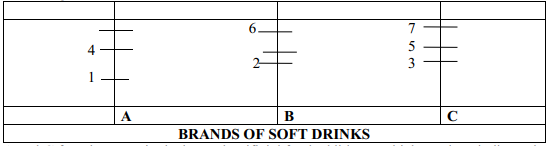 |
 |
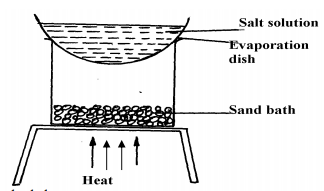 |
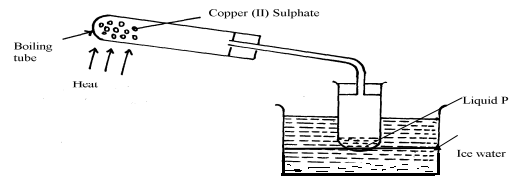 |
 |
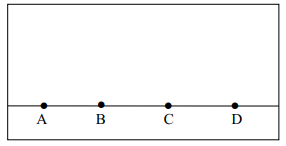 |
| Liquid | L3 | L4 |
| L1 | Miscible | Miscible |
| L2 | Miscible | Immiscible |
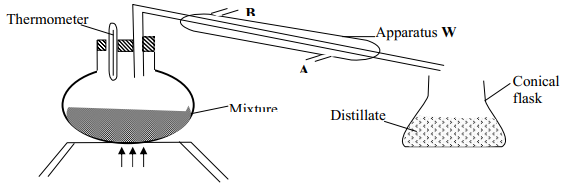 |
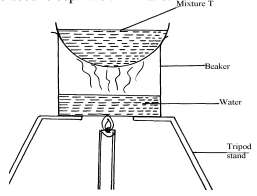 |
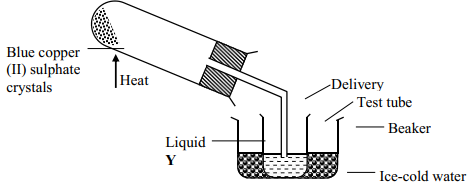 |
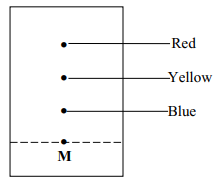 |
| Solid | Cold water | Hot water |
| R | Soluble | Soluble |
| V | Insoluble | Insoluble |
| S | Insoluble | Insoluble |
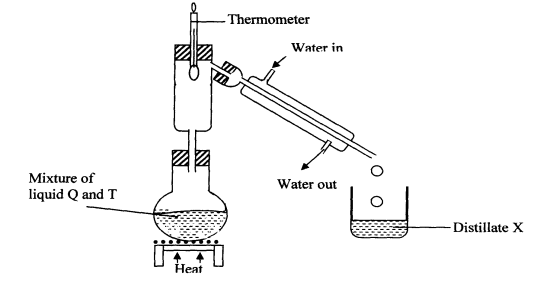 |
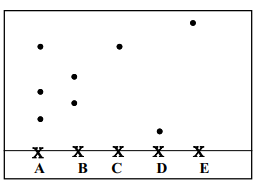 |
| Solid | Water | Alcohol | Ether |
| A | Soluble | Insoluble | Insoluble |
| B | Insoluble | Soluble | Very soluble |
| C | Soluble | Soluble | Insoluble |
| Solution | Observations on indicator |
| A | Methyl orange turns yellow |
| B | Phenolphthalein turns colourless |
| C | Litmus turns purple |
| Solution | PH values |
| V W X Y Z |
2 6.5 11 14 4.5 |
| Indicator | Colour in | |
| Methyl Orange | Acidic Solution | Basic Solution |
| Phenolphthalein | Colourless | |
| Solution | G | H | I | J | K |
| pH | 1.5 | 6.5 | 13.0 | 7.0 | 8.0 |
| MEDICINE | pH VALUES |
| P Q R S |
7.0 5.0 8.0 6.0 |
| Solution | pH |
| P Q R |
14.0 6.8 2.5 |
| Solution | Blue litmus paper | Indicator W |
| Distilled water | ………………….. | Colourless |
| Calcium hydroxide | Blue | Pink |
| Nitric acid | ………………………… | Colourless |
 |
| Indicator | Colour in acidic solution | Basic Solution |
| Methyl orange | Pink | |
| Phenolphthalein | Pink |
| Solutions | pH Values |
| K | 1.5 |
| L | 7.0 |
| M | 14.0 |
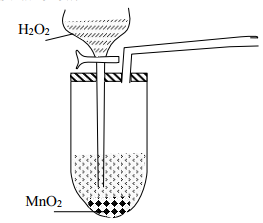 |
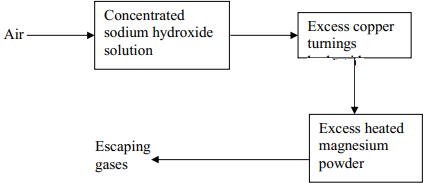 |
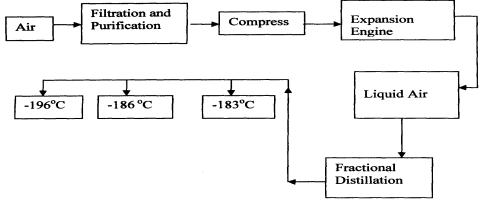 |
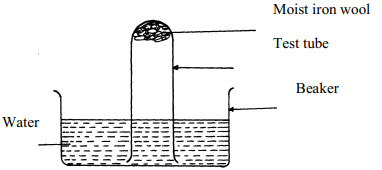 |
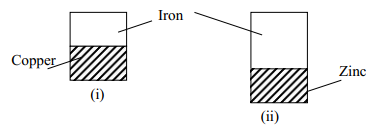 |
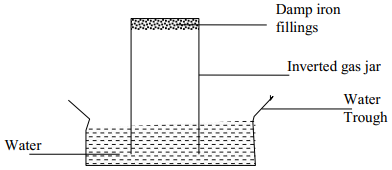 |
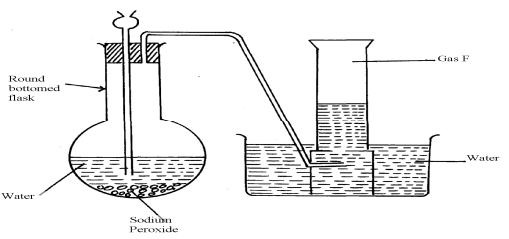 |
 |
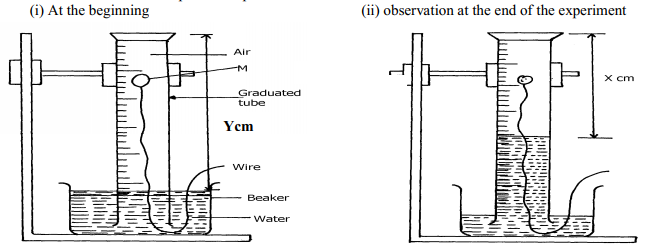 |
access all the content at an affordable rate
or
Buy any individual paper or notes as a pdf via MPESA
and get it sent to you via WhatsApp
