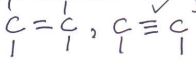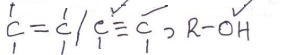CHEMISTRY
PAPER 3
PRACTICAL
INSTRUCTIONS TO CANDIDATES
- Answer ALL the questions in the spaces provided in the question paper.
- You are not allowed to start working with the apparatus for the first 15 minutes of the 2 ¼ hours allowed for this paper. This time is to enable you to read the question paper and make sure you have all the chemicals and apparatus that you may need.
- All working MUST be clearly shown where necessary.
- Mathematical tables and silent electronic calculators may be used.

Questions
- You are provided with:-
- Solution T, 2M Hydrochloric acid.
- Solution P, 0.15M Sodium thiosulphate
- Solution S, Sodium carbonate
- Procedure 1
Measure 20cm3 of 0.15M Sodium thiosulphate (solution P) into a 250cm3 a conical flask. Place the beaker on a white piece of paper with ink mark ‘X’ on it. Measure 20cm3 of 2M hydrochloric acid solution T using a 50cm3 measuring cylinder. Put the acid into the conical flask containing Sodium thiosulphate and immediately start off the stop watch. Determine the time taken for the mark ‘X’ to become invisible /obscured when viewed from above. Repeat the procedure by measuring different volumes of the acid and adding the volumes of the distilled water to complete Table I below.
Table 1
Volume of acid
(cm3)Volume of water
(cm3)Volume of sodium thiosulphate
(cm3)Time taken for mark ‘X’
to be invisible/obscured
(seconds)Reciprocal of time
(sec-1)
1/t20 0 20 18 2 20 16 4 20 14 6 20 12 8 20 10 10 20 - Complete the table above (6 marks)
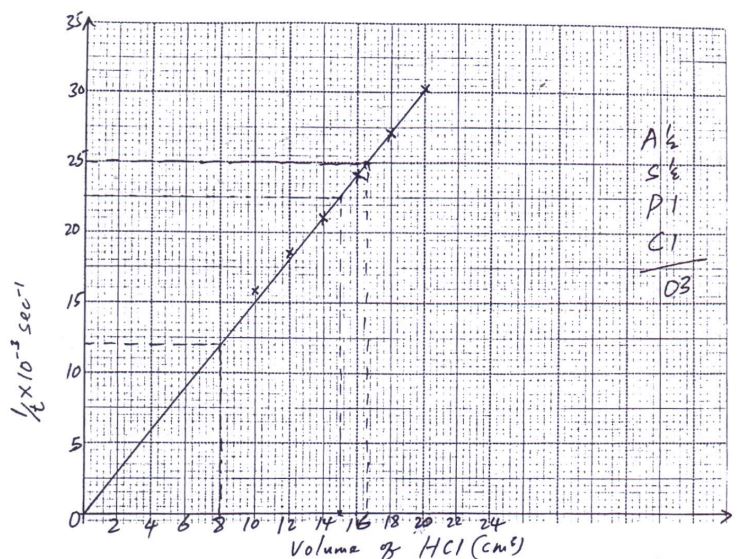
- Plot a graph of 1/t (rate) against volume of acid used. (3 marks)
- Explain the shape of your graph (1 mark)
- From the graph determine
- Time taken for the cross to be obscured/invisible when the volume of the acid is:
- 15cm3 (1 mark)
- 8cm3 (1 mark)
- The volume of the acid used if the time taken for the cross to be obscured/invisible is:
- 40 seconds (1 mark)
- 43 seconds (1 mark)
- Time taken for the cross to be obscured/invisible when the volume of the acid is:
- Procedure 2
Using a 10 cm3 measuring cylinder, place 10 cm3 of solution T into a 250 ml volumetric flask. Add about 200 cm3 of distilled water. Shake well. Add more distilled water to top up to the mark. Label this solution U. Fill the burette with solution U. Using a pipette and pipette filler, pipette 25 cm3 of solution S into a conical flask. Add 3 drops of Phenolphthalein indicator and titrate with solution U.
− Record your results in the table.
− Repeat the titration two more times and complete the table.
Table 2
(4 marks)I II III Final burette reading (cm3) Initial burette reading (cm3) Volume of solution U(cm3) added - Determine the:-
- Average volume of solution U used. (1 mark)
- Moles of the acid in the average volume of solution U used. (2 marks)
- Concentration of solution S in moles per litre. (2 marks)
- Determine the:-
-
- Put a spatula end-full of solid Q into a boiling tube and add about 10cm3of distilled water. Shake the mixture well. Divide the resultant solution into 4 equal portions.
Observations Inferences -
- The solution is suspected to contain ammonium ions. Using calcium hydroxide solid and red litmus paper provided, describe how you would confirm presence of the ammonium ions.
Observations Inferences - Carry out the actual test as described in (b) (i) above.
Observations Inferences
- The solution is suspected to contain ammonium ions. Using calcium hydroxide solid and red litmus paper provided, describe how you would confirm presence of the ammonium ions.
- To the second portion, add 4 drops of hydrogen peroxide solution. Test the gas produced using a glowing splint.
Observations Inferences -
- The solution is also suspected to contain sulphite ions. Using Barium nitrate solution and dilute hydrochloric acid solution, describe how you would confirm presence of the sulphite ions.
Observations Inferences - Carry out the actual test as described in (d) (i) above.
Observations Inferences
- The solution is also suspected to contain sulphite ions. Using Barium nitrate solution and dilute hydrochloric acid solution, describe how you would confirm presence of the sulphite ions.
- Put a spatula end-full of solid Q into a boiling tube and add about 10cm3of distilled water. Shake the mixture well. Divide the resultant solution into 4 equal portions.
- You are provided with solid R. Carry out the tests below and record your observations and inferences in the spaces provided.
- Place one third of solid R on a metallic spatula. Burn it in a non-luminous flame of the Bunsen burner.
Observations Inferences - Place the remaining solid in a test-tube. Add about 6cm3of distilled water and shake the mixture well. Retain the solution for the next procedure.
Observations Inferences - To about 2cm3 of the solution, add 2 drops of acidified potassium manganate (VII).
Observations Inferences - To about 1cm3 of the solution, add 3 drops of acidified potassium dichromate (VI) and warm.
Observations Inferences - To about 2cm3 of the solution, add 1g of sodium hydrogen carbonate.
Observations Inferences
- To about 2cm3 of the solution, add 2 drops of acidified potassium manganate (VII).
- Place one third of solid R on a metallic spatula. Burn it in a non-luminous flame of the Bunsen burner.

Confidentials
Apart from the usual laboratory fittings, each student should have the following;
- About 0.5g of Solid Q in a stoppered container
- About 0.5g of Solid R in a stoppered container
- 100cm3 of solution S
- 100cm3 of solution T
- 100cm3 of solution P
- Distilled water.
- About a spatula end-full of solid Calcium hydroxide
- Red litmus paper
- Three 250ml conical flasks
- One burette 0 – 50ml
- One pipette 25ml
- One 50ml measuring cylinder
- One 10ml measuring cylinder
- One 250cm3 volumetric flask
- Phenolphthalein indicator
- Labels (2)
- Stop watch
- Two boiling tubes
- One metallic spatula
- Five test tubes on a test-tube rack
- Wooden splint
- Test tube holder
The student should also get access to;
- 10% Hydrogen peroxide (freshly prepared + dropper).
- 2M Barium nitrate solution + dropper.
- 0.5M Hydrochloric acid + dropper.
- Source of heat.
- Sodium hydrogen carbonate
- Acidified Potassium manganate (VII)
- Acidified Potassium dichromate (VI)
NOTES
- Solid Q is Hydrated ferrous ammonium sulphate.
- Solid R is Maleic acid
- Solution S is prepared by weighing exactly 4.8g of sodium carbonate dissolve it to make 1dm3 of solution.
- Solution T is prepared by weighing exactly 172cm3 of hydrochloric acid (35-37% sp.gr 1.18) and dissolving to make 1dm3 of solution.
- Solution P is prepared by weighing exactly 37.2g of sodium thiosulphate pentahydrate and dissolving to make 1dm3 of solution.

Marking Scheme
- You are provided with:-
- Solution T, 2M Hydrochloric acid.
- Solution P, 0.15M Sodium thiosulphate
- Solution S, Sodium carbonate
- Procedure 1
Measure 20cm3 of 0.15M Sodium thiosulphate (solution P) into a 250cm3 a conical flask. Place the beaker on a white piece of paper with ink mark ‘X’ on it. Measure 20cm3 of 2M hydrochloric acid solution T using a 50cm3 measuring cylinder. Put the acid into the conical flask containing Sodium thiosulphate and immediately start off the stop watch. Determine the time taken for the mark ‘X’ to become invisible /obscured when viewed from above. Repeat the procedure by measuring different volumes of the acid and adding the volumes of the distilled water to complete Table I below.
Table 1
Volume of acid
(cm3)Volume of water
(cm3)Volume of sodium thiosulphate
(cm3)Time taken for mark ‘X’
to be invisible/obscured
(seconds)Reciprocal of time
(sec-1)
1/t20 0 20 33 0.0303 18 2 20 37 0.0270 16 4 20 41 0.0244 14 6 20 47 0.0213 12 8 20 57 0.0175 10 10 20 63 0.0159 - Complete the table above (6 marks)
- Plot a graph of 1/t (rate) against volume of acid used. (3 marks)
- Explain the shape of your graph (1 mark)
Increase in volume of HCl increases rate of reaction (1/t). This is due to increase in number of reacting particles hence more successful collisions. - From the graph determine
- Time taken for the cross to be obscured/invisible when the volume of the acid is:
- 15cm3 (1 mark)
1/t = 0.0225
t= 44.44sec
must be shown on graph - 8cm3 (1 mark)
1/t = 0.0120
t= 83.33 sec
Must be shown on graph
- 15cm3 (1 mark)
- The volume of the acid used if the time taken for the cross to be obscured/invisible is:
- 40 seconds (1 mark)
1/t = 0.025
v= 16.6cm3 - 43 seconds (1 mark)
1/t = 0.0233
V= 15.6cm3
- 40 seconds (1 mark)
- Time taken for the cross to be obscured/invisible when the volume of the acid is:
- Procedure 2
Using a 10 cm3 measuring cylinder, place 10 cm3 of solution T into a 250 ml volumetric flask. Add about 200 cm3 of distilled water. Shake well. Add more distilled water to top up to the mark. Label this solution U. Fill the burette with solution U. Using a pipette and pipette filler, pipette 25 cm3 of solution S into a conical flask. Add 3 drops of Phenolphthalein indicator and titrate with solution U.
− Record your results in the table.
− Repeat the titration two more times and complete the table.
Table 2
(4 marks)I II III Final burette reading (cm3) 15.0 15.0 15.0 Initial burette reading (cm3) 0.0 0.0 0.0 Volume of solution U(cm3) added 15.0 15.0 15.0 - Determine the:-
- Average volume of solution U used. (1 mark)
15.0 + 15.0 +15.0 = 45.0
3 3
15.0cm3 - Moles of the acid in the average volume of solution U used. (2 marks)
M₁V₁ = M₂V₂
M₂ = 10 x 2/250
= 0.08M
Moles used = 0.08 x value in a(i)
1000 - Concentration of solution S in moles per litre. (2 marks)
Na2CO3(aq) + 2HCl(aq) → 2NaCl(aq) + CO2(g) + H2O(l)
moles of Na2CO3 = Ans II/2
Molarity = moles of Na₂CO₃ x 1000
25
=Ans IV
- Average volume of solution U used. (1 mark)
- Determine the:-
-
- Put a spatula end-full of solid Q into a boiling tube and add about 10cm3of distilled water. Shake the mixture well. Divide the resultant solution into 4 equal portions.
Observations Inferences Solid A dissolves, forming a plae green solution Fe2+ , Cu2+ suspected -
- The solution is suspected to contain ammonium ions. Using calcium hydroxide solid and red litmus paper provided, describe how you would confirm presence of the ammonium ions.
Observations Inferences To the 1st portion ass little Ca(OH)2 and warm.
Test gas using moist red litmus paper
Colourless gas changes moist red litmus paper to blue - Carry out the actual test as described in (b) (i) above.
Observations Inferences Colourless gas turns moist red litmus paper to blue NH4+
- The solution is suspected to contain ammonium ions. Using calcium hydroxide solid and red litmus paper provided, describe how you would confirm presence of the ammonium ions.
- To the second portion, add 4 drops of hydrogen peroxide solution. Test the gas produced using a glowing splint.
Observations Inferences -Pale green solution turns brown
- Colourless gas relights a glowing splintO2 gas
Fe2+
(must have appeared in (a)) -
- The solution is also suspected to contain sulphite ions. Using Barium nitrate solution and dilute hydrochloric acid solution, describe how you would confirm presence of the sulphite ions.
Observations Inferences To the third portion add 3 drops of Ba(NO3)2
followed by 3 drops of HCl (aq)White ppt soluble on additon of HCL(aq) - Carry out the actual test as described in (d) (i) above.
Observations Inferences White ppt insoluble on adding of HCl(aq) SO42-present
- The solution is also suspected to contain sulphite ions. Using Barium nitrate solution and dilute hydrochloric acid solution, describe how you would confirm presence of the sulphite ions.
- Put a spatula end-full of solid Q into a boiling tube and add about 10cm3of distilled water. Shake the mixture well. Divide the resultant solution into 4 equal portions.
- You are provided with solid R. Carry out the tests below and record your observations and inferences in the spaces provided.
- Place one third of solid R on a metallic spatula. Burn it in a non-luminous flame of the Bunsen burner.
Observations Inferences Solid R melts and turns with a yellow sooty/ smoky flame
- Place the remaining solid in a test-tube. Add about 6cm3of distilled water and shake the mixture well. Retain the solution for the next procedure.
Observations Inferences Solid R dissolves to form a colourless solution Polar organic compound - To about 2cm3 of the solution, add 2 drops of acidified potassium manganate (VII).
Observations Inferences Purple KMnO4 changes to colourless
(decolourises)
- To about 1cm3 of the solution, add 3 drops of acidified potassium dichromate (VI) and warm.
Observations Inferences Orange K2Cr2O7 turns green R-OH present - To about 2cm3 of the solution, add 1g of sodium hydrogen carbonate.
Observations Inferences Fizzing/ Effervesence
bubbles of colourless gasH+, R-COOH, H3O+
- To about 2cm3 of the solution, add 2 drops of acidified potassium manganate (VII).
- Place one third of solid R on a metallic spatula. Burn it in a non-luminous flame of the Bunsen burner.
Download Chemistry Paper 3 Questions and Answers with Confidentials - Royal Exam Series Post Mock Trial Exams 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students