INSTRUCTIONS TO CANDIDATES
- Answer ALL the questions in the spaces provided.
- Mathematical tables and silent electronic calculators may be used.
- All working MUST be clearly shown where necessary.
FOR EXAMINER’S USE ONLY
|
Questions |
Maximum score |
Candidates score |
|
1 |
10 |
|
|
2 |
13 |
|
|
3 |
11 |
|
|
4 |
12 |
|
|
5 |
11 |
|
|
6 |
14 |
|
|
7 |
9 |
|
|
Total score |
80 |
|

QUESTIONS
-
- What is an electrolyte (1mk)
- State how the following substance conduct electricity
- Molten calcium chloride ( ½ mk)
- Graphite ( ½ mk)
- The diagram below shows a set up that was used to electrolyze molten lead (II) iodide
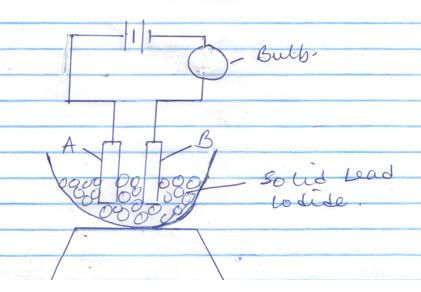
- Name electrodes A and B on the diagram 2mks
- What is omitted in the above set-up for the electrolysis process to take place? (1mk)
- Explain why the bulb lights when the omission is corrected (2mks)
- State the observation made at B(1mk)
- Write the ionic equation for the reaction taking place;2mks
A: ................................................
B: ................................................
-
- Draw the structural formula of . (3 marks)
- 2,3-dimethylpentane
- Pent-2-yne
- 2,3-dimethylbutane
- Study the reaction scheme below and answer the questions that follow.
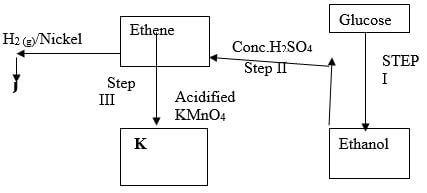
- Name the process in step I (1 mark)
- Give the two conditions necessary in step II (2 marks)
- State the observation made in step III. (1 mark)
- Name compound J. (1 mark)
- Draw the structural formula of compound K. (1 mark)
- Water is added dropwise to calcium carbide in a conical flask.
- Identify the gas produced. (1 mark)
- Write a chemical equation for the reaction that occurs. (1 mark)
- Part of a polymer is required below.
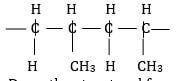
- Draw the structural formula of the monomer of this polymer. (1 mark)
- State one use of this polymer. (1 mark)
- Draw the structural formula of . (3 marks)
- The grid below represents part of the periodic table. Study it and answer the questions that follow. The letters are not the actual symbols of the elements.
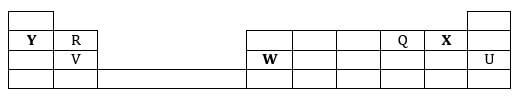
- Select an element whose oxide is amphoteric.(1 mark)
- On the grid indicate with letter J the position of element J which is in period 3 and forms a stable ion J2-. (1 mark)
- Draw a dot-cross diagram to show bonding in the compound consisting of elements V and X only. (2 marks)
- Write an equation to show the formation of an ion of R. (1 mark)
- Which is the least reactive element? Give a reason for your answer. (2 marks)
- Write an equation for the reaction that occurs when element Y is placed in water. (1 mark)
- How does the atomic radius of W compare with that of V? Explain. (2 marks)
- Name the chemical family to which elements R and V belong. (1 mark)
-
- Use the chart below to answer the questions that follow.
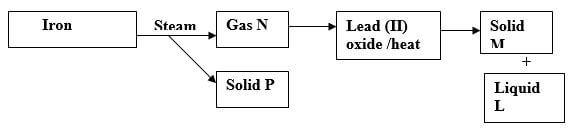
Identify:
Gas N …………………………………………………………………………. (½ mark)
Solid P ……………………………………………………………………….. (½ mark)
Solid M………………………………………………………………………. (½ mark)
Liquid L…………………………………………………………………….. (½ mark) - Name the method that can be used to extract oil from castor oil seeds. (1 mark)
-
- In the method named above, state the property of oil that enables the extraction to take place. (1 mark)
- Describe an experimental procedure that can be used to extract oil from the seeds. (3 marks)
- How is phosphorus stored in the laboratory? Explain your answer. (1 mark)
-
- In the fractional distillation of liquid air water is removed, name two other substances that are removed. (1 mark)
- Why must water be removed? (1 mark)
- State the processes involved in fractional distillation of liquid air. (2 marks)
- Use the chart below to answer the questions that follow.
- Study the flow chart below showing the Solvay process and use it to answer the questions that follow.
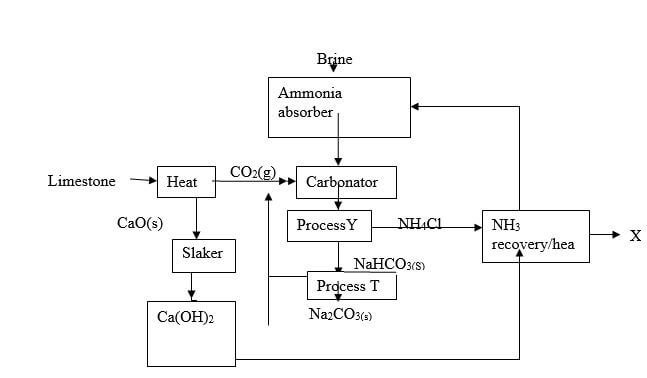
- Write the equation for the reaction producing substance X.(1 mark)
- Name processes Y and T. (1 mark)
Y…………………………………
T…………………………………. - In the carbonator, two reactions take place. Write the two equations for the reactions. (2 marks)
- Explain why the Solvay process is said to be one of the most efficient industrial process. (1 marks)
- 16.8g of sodium hydrogen carbonate are completely decomposed by heating. Calculate;
- the mass of the resulting solid produced. (3 marks)
- the volume in litres of the gas produced at s.t.p (2 marks) (Molar Gas Volume at s.t.p =22400 cm3, Na=23.0, C=12.0, H= 1.0, O=16.0)
- Give two industrial uses of sodium carbonate. (1 mark)
-
- Dissolving of potassium nitrate in water is an endothermic process. Explain the effect of increase in temperature on the solubility of potassium nitrate (2mks)
- The table below shows the solubility of potassium sulphate and potassium chlorate (V) at different temperatures
Temperature oc
0
20
40
60
80
100
Solubility of K2SO4 g/100g of water
8.0
10.0
14.0
17.5
20.0
22.0
Solubility of KClO3 g/100g of H2O
3.0
5.0
15.5
24.0
38.0
53.0
- On the grid provided (graph paper) draw the solubility curves for both salts on the same axis. (Temperature on the X-axis) (3mks)
- A solution of potassium sulphate contains 20g of the salt dissolved in 100g of water at 100ºc. This solution is allowed to cool to 25ºc.
- At what temperature will crystals first appear (1mk)
- What mass of crystals will be present at 25ºc (1mk)
- Which of the two salts is more soluble at 30ºc (1mk)
- Determine the concentration of potassium sulphate in moles per litre when the solubility of the two salts are the same (K=39.0, O=16.0 S = 32.0) (3mks)
- 100g of water at 100ºc contains 19g of potassium sulphate and 19g of potassium chlorate (V). Describe how a solid sample of potassium sulphate at 60ºc can be obtained (1mk)
- Study the solubility curves below and answer the questions that follow.
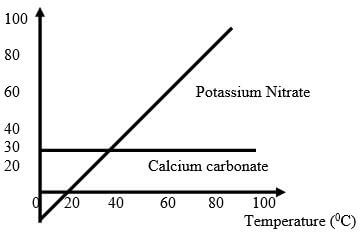
- At what temperature would equal amounts of potassium nitrate and calcium ethanoate dissolve in 100g of water? (1 Mark)
- Explain how you would prepare a saturated solution containing 80g of potassium nitrate in distilled water. (1 Mark)
-
- State two reasons why wood charcoal is not a suitable fuel for cooking. (1 mark)
-
- In the equation below, identify the reagent that acts as a base. Give a reason (2 marks)
H2O2 (aq) + H2O (l) → H3O+ (aq) + H2O (aq) - Distinguish between a strong and weak acid. Give an example of each (2 Marks)
- In the equation below, identify the reagent that acts as a base. Give a reason (2 marks)
- In order to determine the molar enthalpy of neutralization of sodium hydroxide, 50cm3 of 2M sodium hydroxide and 50cm3 of 2M hydrochloric acid both at the same initial temperature were mixed and stirred continuously with a thermometer. The temperature of the resulting solution was recorded after every 15 seconds until the highest temperature of the solution was attained. Thereafter the temperature of the solution was recorded for a further two minutes.
The sketch below was obtained when the temperature of the mixture were plotted against time. Study and answer the questions that follow.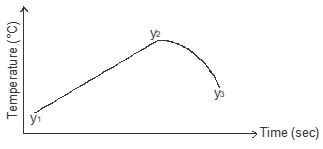
- What is the significance of point y2 (1mark) (1 mark)
- Explain why there is a temperature change between points y1 and y2 (1 mark)
- Explain how the value of temperature rise obtained in this experiment would compare with the one that would be obtained if the experiment was repeated using 50cm3 of 2M methanoic acid instead of hydrochloric acid. (2 marks)

MARKING SCHEME
-
- What is an electrolyte (1mk)
An electrolyte is a substance which when molten or dissolved in water conducts an electric current and gets decomposed by the current. - State how the following substance conduct electricity
- Molten calcium chloride ( ½ mk)
Mobile ions in molten form
Penalise free ions - Graphite ( ½ mk)
- Molten calcium chloride ( ½ mk)
- The diagram below shows a set up that was used to electrolyze molten lead (II) iodide

- Name electrodes A and B on the diagram 2mks
A anode ½
B Cathode ½
- What is omitted in the above set-up for the electrolysis process to take place? (1mk)
heat - Explain why the bulb lights when the omission is corrected (2mks)
solid lead iodide melts/contains molten ions - State the observation made at B(1mk)
purple vapour - Write the ionic equation for the reaction taking place;2mks
A: .2I → I2 (g) + 2e-
B: Pb2 + 2e → Pb (s)
- Name electrodes A and B on the diagram 2mks
- What is an electrolyte (1mk)
-
- Draw the structural formula of . (3 marks)
- 2,3-dimethylpentane
- Pent-2-yne
- 2,3-dimethylbutane
- Study the reaction scheme below and answer the questions that follow.

- Name the process in step I (1 mark)
Fermentation - Give the two conditions necessary in step II (2 marks)
Excess concentrated sulphuric (VI) acid
Temperature: 160ºC-180ºC1 (any value in range) - State the observation made in step III. (1 mark)
Colour of acidified potassium manganate (VII) changes from purple to colourless - Name compound J. (1 mark)
Ethane - Draw the structural formula of compound K. (1 mark)
- Name the process in step I (1 mark)
- Water is added dropwise to calcium carbide in a conical flask.
- Identify the gas produced. (1 mark)
Ethyne - Write a chemical equation for the reaction that occurs. (1 mark)
CaC2(s) +2H2O (l) Ca (OH) 2(aq) + C2H2 (g)
- Identify the gas produced. (1 mark)
- Part of a polymer is required below.
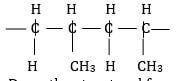
- Draw the structural formula of the monomer of this polymer. (1 mark)
- State one use of this polymer. (1 mark)
Plastic crates and boxes, carpets and plastic bottles.
- Draw the structural formula of . (3 marks)
- The grid below represents part of the periodic table. Study it and answer the questions that follow. The letters are not the actual symbols of the elements.
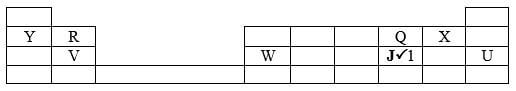
- Select an element whose oxide is amphoteric.(1 mark) W
- On the grid indicate with letter J the position of element J which is in period 3 and forms a stable ion J2-. (1 mark)
(see diagram) - Draw a dot-cross diagram to show bonding in the compound consisting of elements V and X only. (2 marks)
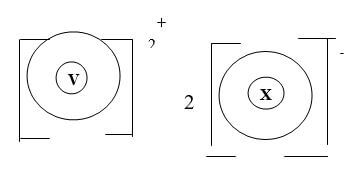
- Write an equation to show the formation of an ion of R. (1 mark)
R → R2+ + 2e- - Which is the least reactive element? Give a reason for your answer. (2 marks)
U;
It has a completely filled outermost energy level therefore neither gains nor loseselectrons//OR has stable electron arrangement. - Write an equation for the reaction that occurs when element Y is placed in water. (1 mark)
2Y(s) + 2H2O (l) → 2YOH (aq) + H2 (g)P - How does the atomic radius of W compare with that of V? Explain. (2 marks)
Atomic radius of W is smaller than that of V. This is because nuclear charge attraction in W is stronger than in V ½while both have same number of occupied energy levels - Name the chemical family to which elements R and V belong. (1 mark)
Alkaline earth metals
-
- Use the chart below to answer the questions that follow.

Identify:
Gas N Hydrogen //H2(g). (½ mark)
Solid P Tri-iron tetraoxide // Fe3O4(s (½ mark)
Solid M Lead // Pb(s)P½ (½ mark)
Liquid L Water //H2O (½ mark) - Name the method that can be used to extract oil from castor oil seeds. (1 mark)
Solvent extraction -
- In the method named above, state the property of oil that enables the extraction to take place. (1 mark)
Oil dissolves in an organic solvent - Describe an experimental procedure that can be used to extract oil from the seeds. (3 marks)
Crush½the seeds in a mortar using a pestle ½.Continue crushing and addpropanone a little at a time½.Decant ½the mixture into an evaporating dish. Leave the mixture of oil and propanone in the sunlight for propanone to evaporate leaving the oil behind
- In the method named above, state the property of oil that enables the extraction to take place. (1 mark)
- How is phosphorus stored in the laboratory? Explain your answer. (1 mark)
Under water½ it smoulders when left in the air.½ -
- In the fractional distillation of liquid air water is removed, name two other substances that are removed. (1 mark)
Dust particles, Carbon (IV) oxide, water vapor - Why must water be removed? (1 mark)
To prevent blocking the pipes in the rest of the system - State the processes involved in fractional distillation of liquid air. (2 marks)
pptn,removal of carbon (IV) oxide by passing the remaining gases through conc.sodium hydroxide½,cooling the remaining part of air to -25oC to remove water vapour,repeated compression and expansion to cool the air to liquid at -200oC½,boiling the liquid mixture in a fractionating column to obtain the fractions.½
- In the fractional distillation of liquid air water is removed, name two other substances that are removed. (1 mark)
- Use the chart below to answer the questions that follow.
- Study the flow chart below showing the Solvay process and use it to answer the questions that follow.

- Write the equation for the reaction producing substance X.(1 mark)
NH4Cl(aq) + Ca(OH)2 (aq) → CaCl2(s) + 2NH3(g) + 2H2O(g)/(l)P - Name processes Y and T. (1 mark)
Y Filtration ½
T Heating/Thermal decomposition ½ - In the carbonator, two reactions take place. Write the two equations for the reactions. (2 marks)
NH3(g) + CO2(g) + H2O(l) → NH4HCO3 (s)
NH4HCO3(s) + NaCl(aq) → NH4Cl(aq) + NaHCO3(s) - Explain why the Solvay process is said to be one of the most efficient industrial process. (1 marks)
Ammonia and carbon (IV) oxide are recycled½ thus minimizing cost½ - 16.8g of sodium hydrogen carbonate are completely decomposed by heating. Calculate;
- the mass of the resulting solid produced. (3 marks)
2NaHCO3(s) → Na2CO3(s) + CO2 (g) +H2O (l)P1
168g of NaHCO3 yield106 g of Na2CO3
16.8 g would yield (16.8/168) ×106P½=10.6 g of Na2CO3P½ - the volume in litres of the gas produced at s.t.p (2 marks) (Molar Gas Volume at s.t.p =22400 cm3, Na=23.0, C=12.0, H= 1.0, O=16.0)
=22400 cm3, Na=23.0, C=12.0, H= 1.0, O=16.0)
1mole of CO2 at s.t.poccupies 22.4 litres
168g of NaHCO3 evolve 22.4 litres of the gas½
16.8 g of NaHCO3 would evolve (16.8/168) ×22.4½=2.24 litres½
- the mass of the resulting solid produced. (3 marks)
- Give two industrial uses of sodium carbonate. (1 mark)
Softening of hard water
Manufacture of glass
- Write the equation for the reaction producing substance X.(1 mark)
-
- Dissolving of potassium nitrate in water is an endothermic process. Explain the effect of increase in temperature on the solubility of potassium nitrate (2mks)
solvent molecules move further apart creating more space between molecules - The table below shows the solubility of potassium sulphate and potassium chlorate (V) at different temperatures
Temperature oc
0
20
40
60
80
100
Solubility of K2SO4 g/100g of water
8.0
10.0
14.0
17.5
20.0
22.0
Solubility of KClO3 g/100g of H2O
3.0
5.0
15.5
24.0
38.0
53.0
- On the grid provided (graph paper) draw the solubility curves for both salts on the same axis. (Temperature on the X-axis) (3mks)
plot ½
curve1/2
plot2 ½
curve2 ½ - A solution of potassium sulphate contains 20g of the salt dissolved in 100g of water at 100ºc. This solution is allowed to cool to 25ºc.
- At what temperature will crystals first appear (1mk)
- What mass of crystals will be present at 25ºc (1mk)
- Which of the two salts is more soluble at 30ºc (1mk)
- Determine the concentration of potassium sulphate in moles per litre when the solubility of the two salts are the same (K=39.0, O=16.0 S = 32.0) (3mks)
solubility of K2SO4 from graph
mass in 100cm3 = (c.v * 1000)/100 1/2
= ANS 1/2MK
Molar mass 174
Ans above /174 1/2
=ans 1/2mk
filter crystals of K2SO4 1/2 and dry between filter papers1/2 - 100g of water at 100ºc contains 19g of potassium sulphate and 19g of potassium chlorate (V). Describe how a solid sample of potassium sulphate at 60ºc can be obtained (1mk)
40ºc - Study the solubility curves below and answer the questions that follow.

- At what temperature would equal amounts of potassium nitrate and calcium ethanoate dissolve in 100g of water? (1 Mark)
add 80g of KNO3 in 100g of water
heat to about 90c then cool to 80c - Explain how you would prepare a saturated solution containing 80g of potassium nitrate in distilled water. (1 Mark)
- At what temperature would equal amounts of potassium nitrate and calcium ethanoate dissolve in 100g of water? (1 Mark)
- On the grid provided (graph paper) draw the solubility curves for both salts on the same axis. (Temperature on the X-axis) (3mks)
- Dissolving of potassium nitrate in water is an endothermic process. Explain the effect of increase in temperature on the solubility of potassium nitrate (2mks)
-
- State two reasons why wood charcoal is not a suitable fuel for cooking. (1 mark)
Bulk
Has low heating value
Its incomplete combustion produce poisonous gases -
- In the equation below, identify the reagent that acts as a base. Give a reason (2 marks)
H2O2 (aq) + H2O (l) → H3O+ (aq) + H2O (aq)
water accepts a proton in the forward reaction - Distinguish between a strong and weak acid. Give an example of each (2 Marks)
Strong acid one that dissociates fully in water to give hygrogen ions
weak acid one that dissociates in water partially to give few hydrogen ions
- In the equation below, identify the reagent that acts as a base. Give a reason (2 marks)
- In order to determine the molar enthalpy of neutralization of sodium hydroxide, 50cm3 of 2M sodium hydroxide and 50cm3 of 2M hydrochloric acid both at the same initial temperature were mixed and stirred continuously with a thermometer. The temperature of the resulting solution was recorded after every 15 seconds until the highest temperature of the solution was attained. Thereafter the temperature of the solution was recorded for a further two minutes.
The sketch below was obtained when the temperature of the mixture were plotted against time. Study and answer the questions that follow.
- What is the significance of point y2 (1mark) (1 mark)
Point of complete neutralisation - Explain why there is a temperature change between points y1 and y2 (1 mark)
Heat was produced during neutralisation hence increase in temperature - Explain how the value of temperature rise obtained in this experiment would compare with the one that would be obtained if the experiment was repeated using 50cm3 of 2M methanoic acid instead of hydrochloric acid. (2 marks)
When methanoic acid is used, there would be a lower temperature rise since some heat is absorbed ½ to completely ionise methanoic acid ½ before neutralisation occurs.
- What is the significance of point y2 (1mark) (1 mark)
- State two reasons why wood charcoal is not a suitable fuel for cooking. (1 mark)
Download Chemistry Paper 2 Questions and Answers - Mathioya Mock 2021 Exams.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

