QUESTIONS
Answer all the questions
-
- What name is given to a compound that contains Carbon and Hydrogen only (1mk)
- Octane is a compound containing Carbon and Hydrogen
- What method is used to obtained Octane from Crude Oil (1mk)
- State one use of Butane (1mk)
- Starting with Potassium Chloride, describe how a pure sample of Lead(II) Chloride can be prepared in the laboratory. (3mks)
- When Magnesium burns in air, it forms a few solid and a grey-green solid. When a few drops of water are added to the mixture a gas that turns red litmus paper blue is evolved. Identify the:
- White solid (1mk)
- Gas evolved and state its use
Name of the gas ………………………. (1mk)
Uses of the gas ………………………… (1mk)
- Iron is extracted from its ore by the blast furnace process.
- Name one ore from which iron is extracted. (1mk)
- One of the impurities in iron is removed in the form of calcium silicate. Write an equation the reaction in which calcium silicate is produced. (1mk)
- Give one use of iron (1mk)
- During the electrolysis of Aqueous Lead (II) Chloride using Lead electrolysis, a current of 0.5 amperes was passed through the cell for 5 hours.
- Write an ionic equation for the reaction that took place at the anode. (1mk)
- Determine the change in mass of the anode which occurred as a result of the electrolysis process (Pb = 207, 1 Faraday = 96,500 Coulombs.) (2mk)
-
- Distinguish between nuclear fission and nuclear fussion. (2mk)
- Radio-active emits three different particles. Give the symbol of the particle with the highest mass (1mk)
- An element Q has two Isotopes Q – 36 and Q – 40 which occur in the ratio Y:4. Given that Relative Atomic Mass of Q is 37.25. Find the value of Y. (3mks)
- The grid below represents part of the periodic table. Study the information and answer the questions that follow. The letters do not represent the actual symbol of the elements.
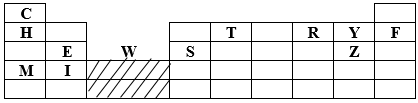
- How does the atomic radius of T compare with that of Y. (1mk)
- Write an equation for the reaction that would occur between E and Z. (1mk)
- Which element would form a trivalent cation? (1mk)
-
- State Graham’s law of diffusion (1mk)
- A volume of 120cm3 of Nitrogen gas diffused through a Membrane in 40seconds, how long will 240cm3 of Carbon (iv) Oxide diffuse through the same Membrane. (N = 14, C = 12, O = 16) (2mks)
- Use the information below on solubility to answer questions that follow
A mixture containing 30g of Potassium Chlorate and 30g of Sodium Carbonate in 100g of water at 80ºC was cooled to 20ºC. Some Crystals are formed.Salt
Solubility in g/100g of H2O
80ºC
20ºC
Na2Co3
80
31
KClO3
55
12
- Which of the two salts crystallized out and by how much? (2mks)
- Name the method used to obtain crystals in the above salts. (1mk)
- Name the salt that will be unsaturated at 20ºC (1mk)
- Study the flow chart below and answer the questions that follows.
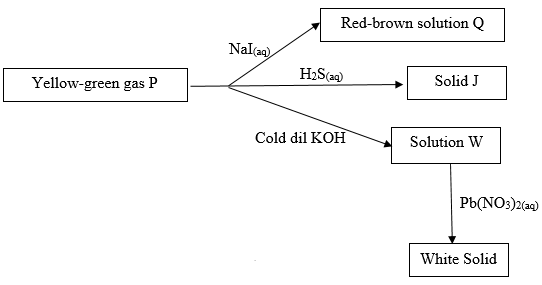
Identify- Solid J (1mk)
- Solution Q (1mk)
-
- Half-life of a radio-active element is 30 days. Define the term half-life. (1mk)
- Calculate the time required for its mass to reduce to 37.5 %(2mks)
- A compound was analysed and found to contain 24.27% Carbon, 4.08% Hydrogen and the rest is Oxygen. If the molar mass of the compound is 99. Determine the molecular formula of the compound. (C = 12, H = 1, O = 16) (3mks)
- The diagram was used to prepare and collect Sulphur (iv) Oxide gas
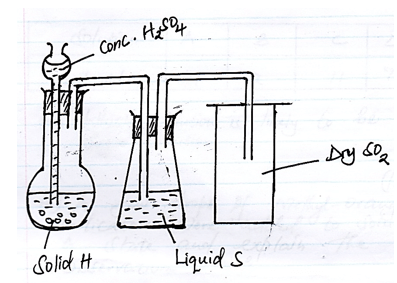
- Identify Solid H (1mk)
- State two properties of SO2 that makes possible to be collected in method shown. (1mk)
- What are the Optimum conditions of conversion of SO2 to SO3 (1mk)
- The table below shows pH values of solutions A, B, C and D.
Solution
A
B
C
D
pH Value
14
2
11
7
- Which solution is likely to be sugar solution (1mk)
- A few drops of Methyl-Orange indicator were added to solution A. State and explain the observations made. (2mks)
-
- Explain why the metals Magnesium and Aluminium are good conductors of electricity. (1mk)
- Other than cost, give two reasons why aluminium is used for making electric cables while Magnesium does not. (2mks)
- The below graph shows a heating curve for substance W. Study and answer the questions that follow
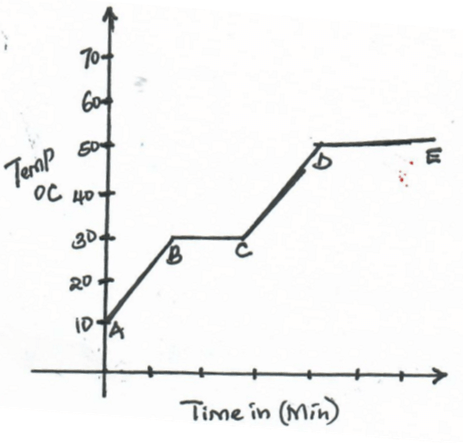
- What is the melting point of substance W.? (1mk)
- In terms of kinetic theory of matter, explain what happened to the particles in region CD (2mks)
-
- Name the catalyst used for the catalytic oxidation of ammonia during Ostwald process. (1mk)
- State and explain the observations made when aqueous ammonia is added to copper (ii) sulphate solution drop wise till in excess. Use equations if necessary. (2mks)
- In an experiment, Carbon(iv) Oxide gas was passed over heated coke and the gas produced collected as shown in the diagram below;
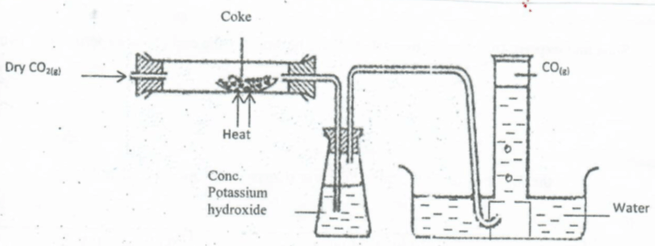
- Write an equation for the reaction that took place in the combustion tube. (2mk)
- Name another substance that can be used instead of potassium hydroxide. (1Mk)
- Hydrogen can be prepared by reacting Zinc with dilute hydrochloric acid.
- Write an equation for the reaction. (1mk)
- Name an appropriate dry agent for hydrogen gas. (1mk)
- Explain why copper metal cannot be used to prepare hydrogen gas. (2mks)
- 25.0 cm3 of a solution of Potassium Carbonate neutralized 26.8cm3 of 0.1M hydrochloric acid. Calculate the Molarity of the carbonate. (3mks)
- At 200 C and 760mmHg pressure, NO2 and N2O exists in equilibrium as shown in the equation below.
2NO2(g) ⇌ N204(g) ∆H= -Ve
Brown pale yellow
State and explain the observations that would be made when;- A syringe containing the gaseous mixture is immersed in ice-cold water.(2mks)
- The volume of the gaseous mixture in a syringe is reduced. (1mk)
- A drop of silver nitrate solution on a glass rod was brought to the mouth of a gas jar containing hydrogen chloride as shown below;
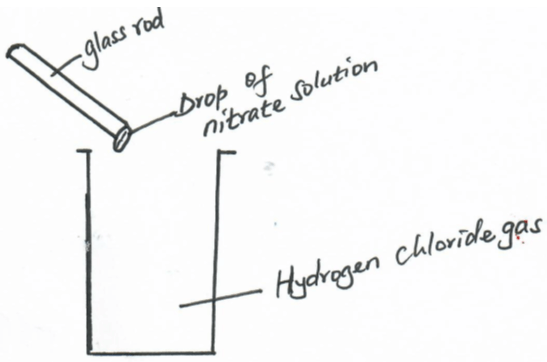
- State and explain the observations made. (2mks)
- Write an ionic equation to represent the reaction above. (1mk)
-
- Give the systematic name of the following compound, (1mk)
- CH3COOCH2CH2CH3
- Alkanoic acid can be prepared through oxidation of alcohols by strong oxidizing agent. Name two oxidizing agents.
- ………………………… (1/2mk)
- ………………………… (1/2mk)
- The structure below represents a pleasant-smelling compound;
CH3-CH2-CH2-COO-CH2CH3
Give the names of the two organic compounds that can be used in its preparation;- ………………………… (1/2mk)
- ………………………… (1/2mk)
- Give the systematic name of the following compound, (1mk)
- Two measuring cylinders were each filled with oxygen and placed in position shown below. After 5 minutes, a glowing splint was then introduced into the mouth of each measuring cylinders. State and explain what was observed in each gas jar (3mks)
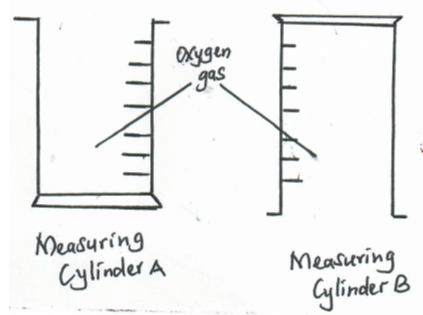
- Use bond energy value given below to answer questions that follow;
Bonds
Bond energy KJM/mol
H-H
432
C=C
610
C-C
346
C-H
413
- Determine the enthalpy change for conversion of butene to butane by hydrogenation. (2mks)
- Sketch an energy level diagram for above enthalpy change. (1mk)
- Study the information in the table below and answer the question that follow. (The letters do not represent the actual symbols of the elements).
Element
Electron configuration
Ionization energy KJ/mol
X
2,8,8,1
418
Y
2,8,1
494
Z
2,1
519
- Give the general name to which the elements belong. Explain (1mk)
- Explain why element Z has the highest ionization energy. (1mk)
MARKING SCHEME
-
- Hydro-Carbon (1mk)
- fractional distillation (1mk)
- Used as a fuel (1mk
- Dissolve KCL in water to form KCL solution. Then react KCL with Pb(NO3)(aq), the salt will react to form PbCL2 and KNO3. Filter to collect PbCL2 as a residue and KNO3 as filtrate. Dry the residue between two filter paper. (3mks)
-
- Magnesium Nitrate (Mg3N2) (1mk)
- Ammonia (NH3) (1mk)
-
- magnetite, haematite, (1m)
- CaO(s) + SiO2(s) → CaSiO3(s)
- Making steel, making alloys.
- Anode
- 2CL- (aq) + 2e- → CL2(g) (1mk)
- Q = IT
= 0.5 × 5 × 3600
= 9000 colu (1mk)
1 mole → 96,500C
? → 9,000C
1 × 9000 = 0.093Mole (½ mk)
96,500
Mass = 0.093 × 207
= 19.3g (½ mk)
-
- Nuclear fussion – Splitting up of heavy nucleid to form small nucleid with absorption of energy. (1mk)
Nuclear fission – Joining of simple nucleid to form a heavy nucleid with a release of energy. (1mk) - Helium particle 42He (1mk)
- Nuclear fussion – Splitting up of heavy nucleid to form small nucleid with absorption of energy. (1mk)
- R.A.M. = (36 × y) + (40 × 4) (1mk)
y + 4
37.25(y + 4) = 36y + 160
37.25y = 36y + 160 – 149
37.25 – 36 =160-149
1.25y = 11 (1mk)
Y = 11/1.25
≈ 8.8
≈ 9 (1mk) -
- T has a layer atomic radius compared to Y since Y has a greater nucleus pull hence small atomic radius. It has more protons than T. (1mk)
- E2+ + Z-1 → E1Z2 or
Mg(s) + CL2(g) → MgCL2(s) - Element S
-
- The rate of diffusion of a gas is inversely proportional to the square root of its density at constant temperature and pressure.
- V1 – 120cm3 V2 – 240cm3
T1 – 40Sec T2 - ?
N2 – 28 CO2 – 44
R1/R2 √(MMR2/MMR1)
(120/40)/(240/T2)=√(44/28)
(B/240)/T2= √1.57
(B/240)/T2 = 1.25
3 = 1.25 × 240
T2 = 1.25 × 240
3
T2 = 100.24Sec
-
- KClO3 (1mk)
30 – 12 = 18g (1mk) - Fraction crystallization (1mk)
- Na2CO3 (1mk)
- KClO3 (1mk)
-
- Sulphur (s)
- Iron (II) Iodide solution
- PbCL2/Lead (II) Chloride
-
- The time taken for a radio-active substance to disintegrate to half its original mass.
- 45 days
- Element C H O
%mass 24.27 4.08 71.65
R.A.M 12 1 16
No. of moles 24.27 4.08 71.65
12 1 16
2.02 4.08 4.4
Mole ratio 2.02 4.08 4.4
2.02 2.02 2.02
1 2 2
E.F.: C1H2O2 E.F.M. = 46
M.F. = 99 (M.F.M)
46 (E.F.M)
= 2
M.F. = 2(CH2O2)
= C2H4O4 -
- Na2SO3 – Sodium Sulphite
- Denser than air
- Does not react with liquid S
-
- Catalyst (V2O5)
- Temp. 450ºC
- Pressure 2-3 atm
Any
-
- Solution D (1mk)
- Turns to pink; Solution A is strong base (2mks)
-
- They have mobile electrons (1mk)
- Light (1mk)
Does not react with Weather conditions++
-
- 30ºC
- Particles gain energy as temperature rises. The energy absorbed is used to weaken the forces holding the particles together.
-
- Platinium-rhodiun
- Pale blue solution form, the deep blue solution
-
- CO2 +C(g)→2CO(g)
- Conc. NaOH/ sodium hydroxide.
-
- Zn(s) + 2 HCl(aq) →Zncl2(aq) +H2(g)
- Conc. H2SO4/Conc. Sulphuric(vi) acid.
- Copper does not react with dilute acids.
- 1000→0.1 moles
26.8→?
= (0.1×26.8)
1000
=0.00268
Equation K2CO3 +2HCl(aq)→KCl(aq)+CO2(g)+H2O(l)
2mol. →1mol K2CO3
Therefore 0.00268→? =(1×0.00268)
2
=0.00134moles
Thus 0.00134 moles →25.0cm3
? ←1000cm3
=(0.00134×1000)
(25 )
=0.0536M -
- Equilibrium shifts to the left ie more of pale yellow decomposed to form brown.
- Equilibrium will shift to the right ie more of brown to form pale-yellow ie (N2O4)
-
- A white ppt is formed . This is due to formation of Agcl ie
AgNO3+HCL(aq) →AgCl(s) + HNO3(aq) - Ag+ (aq) +Cl- (aq) →AgCl(s)
- A white ppt is formed . This is due to formation of Agcl ie
-
-
- propylethanoate
- Acidified KMnO4/Acidified K2Cr2O7
-
- CH3CH2CH2COOH→ butanoic acid
- CH3CH2OH → Ethanol
-
- In A the glowing splint relight
B the splint did not relight
Because : In B the Oxygen had already excaped due to its density. -
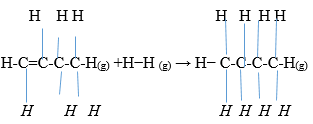
-
- Alkali metals. They have one electron in their outer energy level.
- It has only 2 energy levels thus its electrons are close to the nucleus to remove great amount of energy is required.
Download Chemistry Paper 1 Questions and Answers - Kigumo Mocks 2021 Exams.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

