Questions
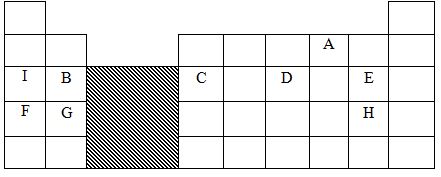
- The grid below shows part of the periodic table. Study it and answer the questions that follow. The letters do not represent the true symbols of the elements
- Which element forms an ion of charge - 2? Explain your answer 2marks
- What is the nature of the oxide formed by element C? 1mark
- How does the reactivity of H compare with that of E? Explain. 2marks
- Write the chemical equation for the reaction between B and chlorine? 1mark
- Explain how the atomic radii of the following compare; 2marks
- F and G
- B and G
- The oxides of B and D are separately dissolved in water. State the effect of each product on litmus paper. 2marks
- 20cm3 of a solution of a hydroxide of I completely neutralizes 17.5cm3 of 0.5M sulphuric (VI) acid. Calculate the concentration in moles/litre of solution of the hydroxide of I 3marks
-
- Sulphur occurs naturally in two different forms called allotropes;
- What are allotropes? 1mark
- The two allotropes of sulphur are stable at different temperatures, as shown in the equation below.
Above 95.50C
Rhombic sulphur ⇌ Monoclinic sulphur
Below 95.50C
Give a name to the temperature 95.50C 1mark
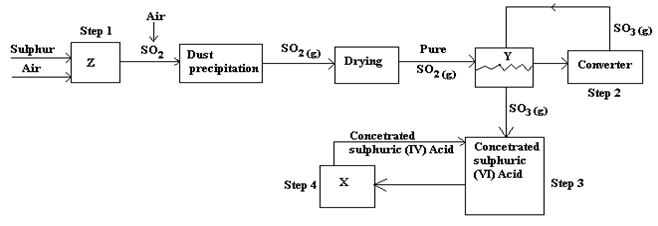
- Below is a flow chart diagram for the contact process for the manufacture of sulphuric (VI) acid.
- Give the name of chambers labeled 1 ½ mark
X
Y
Z - State the three conditions in the converter. 1 ½ mark
- Explain why gases are passed through ; 2marks
- I – The dust precipitator and drying power
- II- The chamber labeled Y Write the balanced equations for the reactions in;3marks
Step 2:
Step 3:
Step 4:
- Calculate the volume of sulphur (VI) oxide gas in litres that would be required to produce 178kg of Oleum in step 3. (Molar gas volume at s.t.p.=22.4l, H=1, O=16, S=32) 3marks
- Sulphur occurs naturally in two different forms called allotropes;
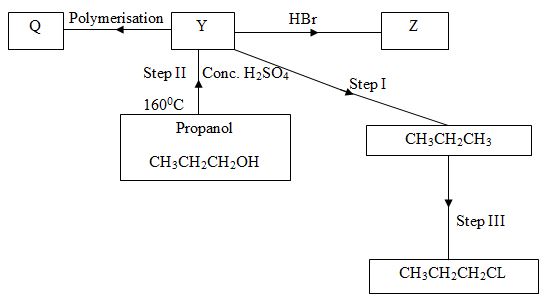
- Below is a scheme of some reactions of propanol. Study it and answer the questions that follow.
- State the reagents and conditions required to effect step I 3marks
- Draw the structural formulae and name product Z. 1mark
- Name product Q 1mark
- Explain how product Y can be distinguished from the product formed after step I has taken place. 2marks
- What name is given to the process in Step II and step III 2marks
Step II
Step III -
- Define the term hydrocarbon 1mark
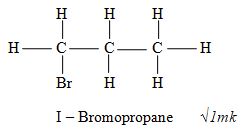
- Draw the structure of 1, 2 – dibromopropane 1mark
-
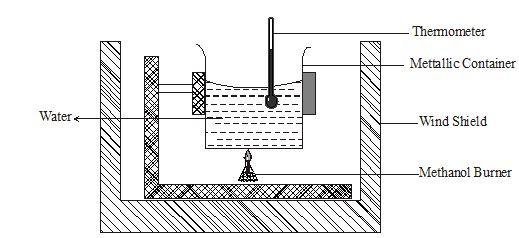
- What is the molar heat of combustion of a substance? (1mark)
- The experiment below was set up to determine the molar heat of combustion of methanol.
The following data was obtained from the above experiment.
Mass of burner + methanol before burning = 62.74g
Mass of burner + methanol after burning = 62.36g Final temperature of water = 38.50C
Initial temperature of water = 23.50C
Volume of water used = 100cm3- From the above results work out the molar heat of combustion of methanol. (3marks)
(Density of water =1g/cm3, C = 12, O=16, H= 1.0)
Specific heat capacity of solution 4.2Kj K-1g K-1) - Write a thermo chemical equation for this reaction. (1mark)
- Explain why the value obtained in (i) above may be lower than the actual value. (1mark)
- From the above results work out the molar heat of combustion of methanol. (3marks)
- Study the data given below
C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(l) ΔH = - 2209 KJmol-1
H2(g) + ½ O2(g) → H2O(l) ΔH = -286KJmol-1
C(s) + O2(g) → CO2(g) ΔH = -406KJmol-1
Use this information to find the heat of formation of propane. (3marks) - What do you understand by the term heating value of a given fuel? (1mark)
- State two factors you consider when choosing a fuel. (1mark)
-
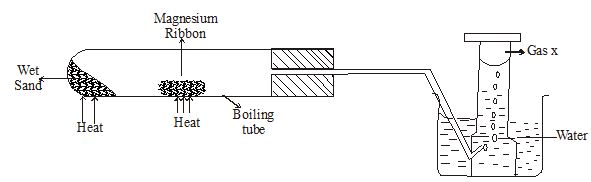
- Magnesium ribbon was reacted with steam as shown in the diagram below.
- State two observations in the boiling tube. (2marks)
- Describe how you test for gas x (2marks)
- State one industrial use of the product formed in the boiling tube at the end of the experiment. (1mark)
-
- Explain what is meant by the term neutralisation. (1mark)
- Starting with 50cm3 of 2M nitric (v) acid, describe how you would prepare crystals of sodium nitrate. (3marks)
- Complete the table below. (1mark)
Indicator Colour in Acidic solution Alkaline solution Phenolphthalein _________ Pink Methyl Orange Pink _________
- When magnesium is burnt in air two reactions take place forming two different compounds. Write down the equations for the two reactions. (2marks )
- Magnesium ribbon was reacted with steam as shown in the diagram below.
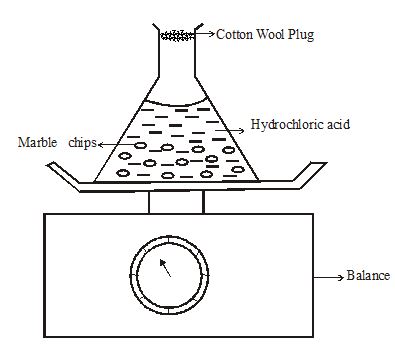
- The set up below is used to measure the change in mass during the course of the reaction between dilute hydrochloric acid (Excess) and marble chips at 220C.
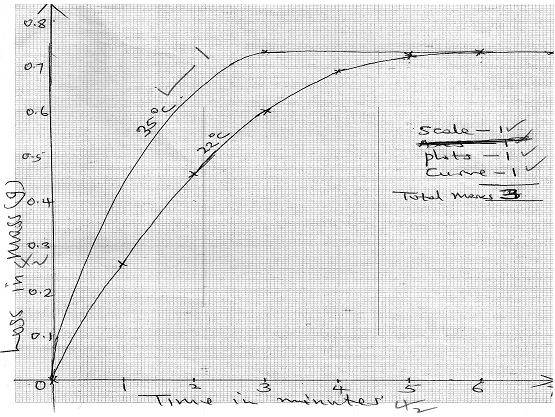
Changes in mass were noted at one minute intervals and were as follows;
Time (Min) 1 2 3 4 5 6 7 Loss in mass (g) 0.26 0.46 0.60 0.69 0.73 0.73 0.73 - Write an equation for the reaction taking place in the flask. (1mark)
- Give a reason why the mass of the flask charged with time? (1mark)
- What is the role of cotton wool at the mouth of the flask? (1mark)
- Explain why it is not advisable to use dilute sulphuric (VI) acid with marble chips in this experiment (1mark)
- Plot a graph of loss in mass (vertical axis) against time. Label the curve 220C (3marks)
- On the same axis in (e) above sketch the graph you would expect to obtain if the experiment was repeated at 350C. Label the curve 350C. (1mark)
- State what would happen if the marble chips were replaced with the same mass of marble powder. Explain your answer. (1mark)
- Determine the volume of carbon (IV) oxide produced if 0.12g of marble chips was reacted with excess dilute hydrochloric acid. (Experiment done at room temperature and pressure. Molar gas volume at r.t.p = 24dm3,Ca = 40.0,O = 16, C = 12.0) (2marks)
- In an experiment ,0.71g of hydrated sodium carbonate (Na2CO3.XH2O) was treated with dilute nitric v acid and the gas evolved was carbon iv oxide which was measured using a syringe at stp.The volume of carbon iv oxide obtained was 56cm3
- Write the equation for the reaction between anhydrous sodium carbonate and dilute nitric v acid (1mk)
- Calculate the number of moles of carbon iv oxide gas collected at s.t.p (molar gas volume at stp=22,400) (2mks)
- Calculate the mass of anhydrous sodium carbonate reacted (3mks)
- Calculate the mass of water in 0.715g of hydrated sodium carbonate (1mk)
- Determine the R.F.M of hydrated sodium carbonate, hence the value of X (3mks)
Marking Scheme
-
- A (√1mk) elements in group (vi) have 6 electrons in the outermost energy level, they react by gaining 2 electrons.√1mk
- Amphoteric Oxide √1mk
- Element E is more reactive than H (√1mk) Elements E and H are non - metals in group (VII) and reactivity decreases down the group √1mk / E is smaller than H and hence has a higher electron affinity therefore more reactive.
- B(s) + Cl2(g) BCl2(s) 1mk
-
- The atomic radius of element F is greater than that of G √1mk / Across period number of protons (nuclear charge increases increasing effective nuclear charge.
- The atomic radius of element G is greater than that of B. √1mk
- Solution of oxide of B changes red litmus paper blue and has no effect on blue litmus paper 1mk while solution of oxide of D changes blue litmus paper red and has no effect on red litmus paper. 1mk
- 2IOH (aq) + H2SO4 (aq) I2SO4(aq) + 2H2O(l) √1mk
2 : 1
Moles of H2SO4 17.5 x0.5 = 0.00875moles √½ mk
1000
Moles of IOH 0.00875 ÷ 2 = 0.004375 moles √½ mk
Molarity of IOH = 1,000 x 0.004375
20
= 0.21875M √1mk
Concentration = 0.21875 moles/litre √½ mk
- A (√1mk) elements in group (vi) have 6 electrons in the outermost energy level, they react by gaining 2 electrons.√1mk
-
-
- Crystalline forms of sulphur √1mk
Or
Existence of sulphur in more than one form in the same physical state.√1mk - Transition temperature √1mk
- Crystalline forms of sulphur √1mk
-
- X - dilution chamber √1 ½ mk
Y- Heat exchanger √1 ½ mk
Z - Burner √1 ½ mk - Vandalism (v) catalyst √1 ½ mk
Temperature – 5000C √1 ½ mk
Pressure – 200atm √1 ½ mk - I – To remove dust particles and water vapour that could otherwise poison the catalyst √1mk
II- Lose heat and pre-heat incoming gases √1mk - Step 2; 2SO2(g) + O2(g) 2SO3(g) √1mk
Step 3: SO3(g) + H2SO4(l) H2S2O7 (l) √1mk
Step 4: H2S2O7(l) + H2O(l) 2 H2SO4(l) √1mk - H2SO4(l) + SO3(g) H2S2O7(l) √½ mk
1 : 1 : 1
1 mole of oleum = 178,000 = 1,000moles
178
1 mole at s.t.p = 22.4L
1,000moles = ? √½ mk
= 1000 x 22.4 = 22,400 litres √1mk
- X - dilution chamber √1 ½ mk
-
-
- Reagent : Hydrogen gas √1mk
Conditions: - Nickel catalyst √1mk
- I50-2500C (temperature) √1mk -
I – Bromopropane √1mk - Polypropene √1mk
- Y decolourisesbromine water √1mk while the product formed after step I has taken place does not √1mk
- Step II – dehydration √1mk
Step III – substitution √1mk -
- A hydrocarbon is a compound that contains carbon and hydrogen only √1mk
-
- Reagent : Hydrogen gas √1mk
-
- Molar heat of combustion is the enthalpy change that occurs when one mole of a substance is burnt completely in oxygen. √ 1 mk
-
- Mass of methanol = 0.38g √ ½ mk
Change in temp. ΔT = 38.5 – 23.5 = 150C
Heat produced. = MCDT
= 100 x 4.2 x 15 √ ½ mk
1000
= 63.1KJ.
Molar mass of ethanol (CH3OH) = 32 √ ½ mk
Molar heat of combustion = 63.1 x 32 √ ½ mk
0.38
= 5313.68 KJ mol-1 - CH3OH(l) + H2O2(g) CO2(g) + 2H2O(l) √ 1 mk ΔH = -5313.68KJmol-1
- Heat is lost to the environment
Hence the value is lower √ 1 mk
- Mass of methanol = 0.38g √ ½ mk
- Equation for formation of propane.
3C(s) + 4H2(g) C3H8(g) √ 1 mk
Heat of formation = 3 (-406) + 4 (-286) + 2209) √ 1 mk
= -1218 - 4576
= - 3585 KJmol-1 √ 1 mk - This is the amount of heat energy given out when a unit mass or unit volume of fuel is completely burned in oxygen. √ 1 mk
- Heating value
Ease and rate of combustion
Availability
Ease of transportation any 2 correct ½ mk each
Ease of storage
Environmental effects
Cost
-
-
- Magnesium burns with a bright white flame √ 1 mk
A white solid is formed √ 1 mk - Place a burning splint √ 1 mk near the mouth of the test tube containing the gas.
A ‘pop’ sound is produced.
This confirms that the gas is hydrogen √ ½ mk - Making lining of furnaces. √ 1 mk
- Magnesium burns with a bright white flame √ 1 mk
-
- This the reaction between a given number of moles of hydrogen ions (H+)√ 1 mk and an equal number of hydroxide (OH-) ions to form water.
- Add 50cm3 of 2M √ 1 mk sodium hydroxide solution.
- Evaporate √ ½ mk the mixture to obtain a saturated solution.
- Leave the saturated solution for some time to crystallize. √ ½ mk
- Filter the crystals and dry then dry then √ ½ mk between two filter papers
-
Indicator Colour in Acidic solution Alkaline solution Phenolphthalein Colourless Pink Methyl Orange Pink Yellow - 2Mg(s) + O2(g) → 2MgO(s) √ 1 mk
3Mg(s) + N2(g) → Mg3N2(s) √ 1 mk
-
-
- CaCO3(s) + 2HCl(aq) CaCl2(aq) + H2O(l) + Co2(g) √ 1 mk
- The carbon (iv) oxide formed escaped into the atmosphere. √ 1 mk
- To prevent acid from spraying out. √ 1 mk
- Forms insoluble salt √ ½ mk of calcium sulphate which forms a coat √ ½ mk on the surface of the marble chips preventing any further reaction. √ 1 mk
- The reactions rate would increase √ ½ mk marble powder provides a larger surface area, more particles are involved in reactions, thus increasing the rate of reaction. √ ½ mk
- CaCO3(s) + 2HCl+(aq) CaCl2(aq) + H2O(l) + CO2(g) √ ½ mk
1mole 2moles 1mole
0.12g → 0.12 x 24dm3 √ 1 mk
100
= 0.0288dm3 √ ½ mk
-
- Na2CO3(s) + 2HNO3(aq) → 2NaNO3(aq) + CO2(g) +H2O(l)
- 1 mole of CO2(g) → 22400cm3
→ 56cm3
56x1/22400=0.0025 moles - Mole ratio Na2CO3 : CO2
1 : 1
? 0.0025moles
0.0025moles of Na2CO3
1Mole of Na2CO3= 106g
0.0025moles = ?
= 0.0025x106= 0.265g
1 - Mass of water= 0.715-0.265=0.45g
- Na2CO3 H2O
Mass 0.265g 0.45g
RFM 106 18
Moles 0.265/106=0.0025 0.45/18=0.025
Mole ratio 0.0025/0.0025=1 0.025/0.0025=10
X=10
- Na2CO3(s) + 2HNO3(aq) → 2NaNO3(aq) + CO2(g) +H2O(l)
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 2 Questions and Answers - 2021 KCSE Eldoret Diocese Mock Exams.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students