Introduction
- Gas laws looks at the relationship between temperature, volume and pressure of gases.

Boyle’s Law
- In this law, temperature of the gas is kept constant.
- Boyle’s law states: the pressure of a fixed mass of a gas is inversely proportional to the volume, provided temperature is constant.
P α 1/V
P = k/V
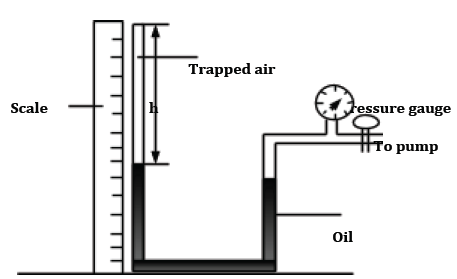
PV = constant. - The following set up can be used to illustrate Boyle’s law
- When pressure is exerted on the oil, the trapped gas (usually air) is compressed and the column h reduces. The pressure is measured using the pressure gauge. Since the cross-section area of the glass tube is uniform, the column h can be taken to represent the volume of the trapped gas (air).
- Several values of pressure, P and volume, h are collected and recorded.
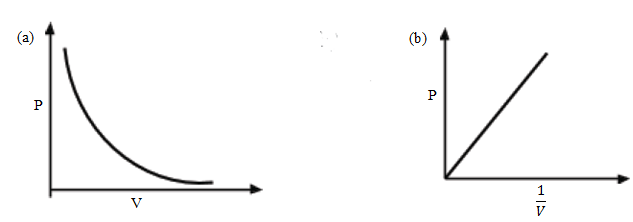
Pressure, P (Pa) Volume, h(cm) 1/v (or 1/hm-1) PV - A graph of pressure against volume is a curve as shown in (a) below
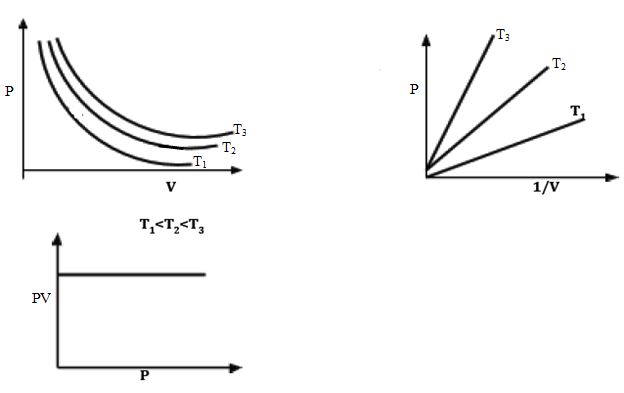
- A graph of P against 1/V is a straight line through the origin as shown in (b) above while a graph of PV against P is a straight line parallel to the x-axis. If the experiment is repeated at different temperatures, similar curves to the above will be obtained. This is shown below:
- Hence for a given mass of a gas, P1V1 = P2V2
Molecular Explanation of Boyle’s Law
- When a gas is put in a closed container, the gas molecules collide with walls of the container generating gas pressure. When the volume of the fixed mass of gas is reduced, the number of collisions per unit time and therefore the rate of change of momentum will increase.
- Consequently the gas pressure is raised. Hence a reduction in volume leads to an increase in the gas pressure.
Example 10.1
- A gas occupies a volume of 1.6cm3 at a pressure of 1.5 × 105 Pa. find the volume it will occupy at a pressure of 9.0 × 105 Pa if the temperature is kept constant.
P1V1 =P2V2
V2 = (1.5 × 105 ×1.6 × 10-6 )/(9.0 × 105 ) = 8.0× 10-7m3 or 0.8cm3 - A column of air 26cm long is trapped by mercury thread 5cm long as shown in (a) below. When the tube is inverted as shown in (b), the air column becomes 30cm long. What is the value of the atmospheric pressure?
In (a), the gas pressure = PAtm + hρg
In (b), the gas pressure = PAtm – hρg
Let the atmospheric pressure be x metres of mercury.
From Boyle’s law, P1V1=P2V2
(x + 0.05)ρg × 0.26=(x − 0.05)ρg × 0.3
0.26x + 0.013=0.3x − 0.015
0.04x=0.028
X=0.028/0.04
=0.7m (or 70cm)
Hence the atmospheric pressure=70cmHg. - The table below shows the results obtained in an experiment to study the variations of the volume of a fixed mass of a gas with pressure at constant temperature:
Fill in the missing values.Pressure, P (cmHg) 60 ... 90 ... Volume, cm3 36 80 ... 40

Charles’ Law
- This law looks at the relationship between temperature and volume of a given mass of gas at constant pressure.
- It is obvious that when a gas is heated it expands i.e. increases in volume.
- The law states: the volume of a fixed mass of a gas is directly proportional to its absolute temperature provided the pressure is kept constant.
i.e. V α T
V=kT or V/T = Constant - The set-up below can be used to verify Charles’ law:
- When the gas (trapped air) is heated in a water bath, it increases in volume. This is showed by an increase in the column h of the trapped air. Thus an increase in temperature of the gas causes an increase in its volume.
- A graph of volume against absolute temperature appears as shown below:
- If the graph is extrapolated, it cuts the x-axis at -273 0 C. at this temperature, the gas is assumed to have a volume equals to zero. This is the lowest temperature a gas can ever fall to and is called the absolute zero . A temperature scale based on the absolute zero is referred to as the absolute or Kelvin scale. On this scale, the temperature must be expressed in Kelvin.
- For a given mass of a gas, V1/T1 = V2/T2
This equation ONLY holds when the temperature is expressed in Kelvin.
Molecular Explanation of Charles’ Law
- When the temperature of a gas is increased, its molecules gain kinetic energy and move faster.
- This increases the rate of collision with walls of the container and hence increased pressure.
- However, since in Charles’ law, pressure must be constant, the volume of the container must be increased accordingly so that the gas molecules can cover larger distance before colliding with the walls of the container.
- This would keep the gas pressure constant although its temperature is raised.
Example 10.2
- A gas occupies a volume of 125cm3 at 15o C and 755mmHg pressure. Find the volume of the gas at a temperature of 25o C if the pressure is constant.
V1/T1 = V2/T2
125/(15+273) = V2/(25+273)
V2 =(125 × 298)/288 =129.34cm3. - To what temperature must 2000cm3 of a gas at 27o C be heated at a constant pressure in order to raise its volume to 2500cm3 ?
V1/T1 = V2/T2
T 2 =(2500 × 300)/2000 =375K or 22o C.

Pressure Law
- Raising the temperature of a fixed mass of a gas at a constant volume increases the average kinetic energy of the gas molecules.
- Pressure law states: the pressure of a fixed mass of a gas is directly proportional to its absolute temperature at a constant volume ;
P α T
P = kT or P/T=k
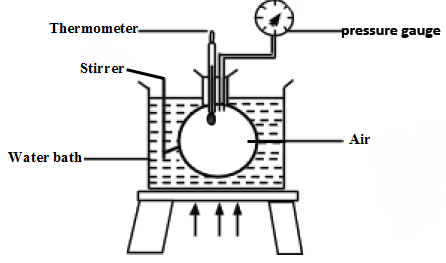
Thus at constant volume, P1/T1 = P2/T2 - The set up below can be used to investigate Pressure law:
- Several values of temperature and the corresponding pressures can be collected and used to plot a graph of pressure against absolute temperature. The graph will appear as shown below:
Example 10.3
- A tin closed with an airtight lid contains air at a pressure of 1.0 × 105 Pa at a temperature of 12o C. If the temperature at which the lid opens is 88o C, determine the pressure attained by the gas.
P1/T1 = P2/T2
P2 = [1.0 × 105 × 361]/285 =126,668.67Pa
The three laws combined can be expressed as;PV/T =constant, k Or simply
P1V1/T1 = P2V2/T2
The above equation is referred to as the equation of state . In general for a fixed mass of a gas, PV/T=a constant. If 1 mole of the gas is used, then;
PV/T= R, where R is the universal gas constant .
Example 10.4
- A gas occupies a volume of 200cm3 at 25o C and 760mmHg. Find its new volume at -23o C and 750mmHg.
P1V1/T1 = P2V2/T2
V2 =[P1V1T2]/P2T1
=[760 × 200 × 250]/[750 × 298] =170cm3
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download GAS LAWS - Form 3 Physics Notes.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students