INSTRUCTIONS TO CANDIDATES
- Write your name and index number in the spaces provided.
- Sign and write the date of examination in the spaces provided.
- Answer all the questions in the spaces provided in the question paper
- You are not allowed to start working with the apparatus for the first 15 minutes of the 2¼ hours allowed for this paper. This time is to enable you to read the question paper and make sure you have all the chemicals and apparatus required.
- All working must be clearly shown where necessary
- Mathematical tables and electronic calculators may be used.
- This paper has 7 printed pages. Check to confirm that it is so.
FOR EXAMINER’S USE ONLY
|
QUESTION |
Max Score |
Candidate Score |
|
1 |
22 |
|
|
2 |
10 |
|
|
3 |
08 |
|
|
TOTAL |
40 |

QUESTIONS
-
- You are provided with:
- Solution A ,containing 39.2g/l of FeSO4(NH4)2SO4.nH2O
- Solution B Containing 3.0g/l of KMnO4.
You are required to determine; - The concentration of solution A in moles per litre
- The number of moles of (n) of water of crystallization in FeSO4(NH4)2SO4.nH2O
- You are provided with:
Procedure
- Fill the burette with solution A.
- Using a pipette filler, pipette 25.0cm3 of solution B into a conical flask and titrate with solution A until a pink colour just appears.
- Record thevolume of solution A used in the table below. Repeat the experiment twice and fill the table.
Table 1
|
Titrations |
1 |
2 |
3 |
|
Final burette reading (cm3) |
|||
|
Initial burette reading (cm3) |
|||
|
Volume of solution A (cm3) |
(4mks)
- Calculate the average volume of solution A used (1mk)
- Determine;
- Concentration of solution B in moles per litre, (1mk)
(K=39,Mn=55,O=16) - Number of moles of solution B used. (1mk)
- Concentration of solution B in moles per litre, (1mk)
- Given that the ionic equation for the reaction is:
MnO4-(aq)+8H+(aq)+5Fe2+(aq) → Mn2+(aq)+5Fe3+(aq)+4H2O (l)
Determine the number of moles of solution A used. (1mk)
Determine the;- Concentration of solution A in mole per litre (1mk)
- Relative formula mass of FeSO4(NH4)2SO4.nH2O (1mk)
- Number of moles of water of crystallization (n) in FeSO4(NH4)2SO4.nH2O (1mk)
b. You are provided with 2.0g of solid R in a boiling tube.
You are required to determine the solubility of solid R at different temperatures.
Procedure
- Using a burette, add 3.0cm3 of distilled water into the boiling tube with solid R.
- Gently heat the boiling tube, while stirring the contents carefully with a thermometer until the crystals of R dissolve completely.
- Remove the boiling tube from the flame and allow the contents to cool while stirring with the thermometer. Note the temperature at which crystals just appear and record it in Table II below.
- Add 2.0cm3 of distilled water from the burette into the boiling tube containing the mixture and repeat steps (ii) and (iii) above.
- Repeat step (iv) three more times.
- Calculate the solubility of solid R in water at the different temperatures and complete table 2.
Table 2
|
Total volume of water added (cm3) |
Temperature at which crystals just appear(oC) |
Solubility of solid R in water (g/100g of water) |
|
3 |
||
|
5 |
||
|
7 |
||
|
9 |
||
|
11 |
(5½ marks)
- On the grid provided, plot a graph of solubility of solid R (vertical axis) against temperature(horizontal axis ) (3 marks)
- From your graph, determine
- The temperature at which 35g of solid R would dissolve in 100cm3 of water.(1mk)
- The solubility of solid R at 50oC. (1mk)
- State how solubility varies with temperature. (½mk)
2. You are provided with solid Q. Carry out the tests below and record your observations and inferences in the spaces provided.
- Place all of solid Q in a clean boiling tube. Add about 10cm3 of distilled water and shake. Divide the resulting solution into 4 equal portions.
Observations Inference (1mk) (1mk) - To the 1st portion, add drops of sodium sulphate solution.
Observations Inference (1mk) (1mk) - To the 2nd portion, add sodium hydroxide solution dropwise until excess.
Observations Inference (1mk) (1mk) - To a 3rd portion, add ammonia solution dropwise until in excess.
Observations Inference (1mk) (1mk) - To the fourth portion, add 2-3 drops of acidified barium nitrate solution.
Observations Inference (1mk) (1mk)
- To the 1st portion, add drops of sodium sulphate solution.
3. You are provided with solid F. Carry out the tests and record your observations and inferences in the spaces provided.
- Place half a spatulaful of solid F in a non-luminous flame to ignite.
Observations Inference (1mk) (1mk) - Place the rest of the solid in a test-tube. Add about 6cm3 of distilled water and shake the mixture well. Divide the solution into 3 portions.
Observations Inference (1mk) (1mk) - To about 2cm3 of the solution, add a spatulaful of sodium hydrogen carbonate.
Observations Inference (1mk) (1mk) - To about 2cm3, add 2 drops of acidified potassium manganate (vii) solution.
Observations Inference (1mk) (1mk) - To another 2cm3, add 2 drops of bromine water.
Observations Inference (1mk) (1mk)

MARKING SCHEME
-
- You are provided with:
- Solution A ,containing 39.2g/l of FeSO4(NH4)2SO4.nH2O
- Solution B Containing 3.0g/l of KMnO4.
You are required to determine; - The concentration of solution A in moles per litre
- The number of moles of (n) of water of crystallization in FeSO4(NH4)2SO4.nH2O
- You are provided with:
Procedure
- Fill the burette with solution A.
- Using a pipette filler, pipette 25.0cm3 of solution B into a conical flask and titrate with solution A until a pink colour just appears.
- Record thevolume of solution A used in the table below. Repeat the experiment twice and fill the table.
Table 1
|
Titrations |
1 |
2 |
3 |
|
Final burette reading (cm3) |
|||
|
Initial burette reading (cm3) |
|||
|
Volume of solution A (cm3) |
CT - 1
DP - 1
AC - 1
PA - 1
FA - 1
05
(4mks)
- Calculate the average volume of solution A used (1mk)
- Determine;
- Concentration of solution B in moles per litre, (1mk)
(K=39,Mn=55,O=16)
3.0
168
=0.01786M - Number of moles of solution B used. (1mk)
25 × Ans (b)(i)
1000
- Concentration of solution B in moles per litre, (1mk)
- Given that the ionic equation for the reaction is:
MnO4-(aq)+8H+(aq)+5Fe2+(aq) → Mn2+(aq)+5Fe3+(aq)+4H2O (l)
Determine the number of moles of solution A used. (1mk)
Ans b(ii) × 5
Determine the;- Concentration of solution A in mole per litre (1mk)
1000 × Ans (c)
ans(a)
Ans (c2) - Relative formula mass of FeSO4(NH4)2SO4.nH2O (1mk)
R.F.M = 39.2
Ans.c (i) - Number of moles of water of crystallization (n) in FeSO4(NH4)2SO4.nH2O (1mk)
294 + 18n = ans(c2)
- Concentration of solution A in mole per litre (1mk)
b. You are provided with 2.0g of solid R in a boiling tube.
You are required to determine the solubility of solid R at different temperatures.
Procedure
- Using a burette, add 3.0cm3 of distilled water into the boiling tube with solid R.
- Gently heat the boiling tube, while stirring the contents carefully with a thermometer until the crystals of R dissolve completely.
- Remove the boiling tube from the flame and allow the contents to cool while stirring with the thermometer. Note the temperature at which crystals just appear and record it in Table II below.
- Add 2.0cm3 of distilled water from the burette into the boiling tube containing the mixture and repeat steps (ii) and (iii) above.
- Repeat step (iv) three more times.
- Calculate the solubility of solid R in water at the different temperatures and complete table 2.
Table 2
|
Total volume of water added (cm3) |
Temperature at which crystals just appear(oC) |
Solubility of solid R in water (g/100g of water) |
|
3 |
66.7 | |
|
5 |
40.0 | |
|
7 |
28.6 @ ½ for solubility | |
|
9 |
22.2 | |
|
11 |
18.2 |
CT - 1
DP - ½
T - ½
AC- 1
03(5½ marks)
- On the grid provided, plot a graph of solubility of solid R (vertical axis) against temperature(horizontal axis ) (3 marks)

P - 1
A - ½
S - ½
C - 1
03 - From your graph, determine
- The temperature at which 35g of solid R would dissolve in 100cm3 of water.(1mk)
Shown from graph ½
Value ½ - The solubility of solid R at 50ºC. (1mk)
From graph - State how solubility varies with temperature. (½mk)
Solubility increaes with increase in temperature
- The temperature at which 35g of solid R would dissolve in 100cm3 of water.(1mk)
2. You are provided with solid F. Carry out the tests below and record your observations and inferences in the spaces provided.
- Place all of solid F in a clean boiling tube. Add about 10cm3 of distilled water and shake. Divide the resulting solution into 4 equal portions.
Observations Inference Dissolves to form a colourless solution -soluble salt
-Cu2+, Fe2+, Fe3+ absent
i)To the 1st portion, add drops of sodium sulphate solution
| Observations | Inference |
| No white ppt | Pb2+, Ba2+, Ca2+ absent (penalize 1/2 for any contradicting) |
ii)To the 2nd portion, add sodium hydroxide solution dropwise until excess
| Observations | Inference |
| White ppt soluble in excess | Al3+, Zn2+ present (penalize 1/2 for Pb2+ present) |
iii)To a 3rd portion, add ammonia solution dropwise until in excess.
| Observations | Inference |
| white ppty insoluble in excess | Al3+ (penalize 1/2 for any other ion) |
iv)To the fourth portion, add 2-3 drops of acidified barium nitrate solution.
| Observations | Inference |
| white ppt | So42- present (penalize 1/2 for any contradicting) |
3. You are provided with solid Q. Carry out the tests and record your observations and inferences in the spaces provided.
i)Place half a spatulaful of solid F in a non-luminous flame to ignite.
| Observations | Inference |
| -melt -burns with yellow sooty flame |
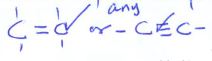 |
ii)Place the rest of the solid in a test-tube. Add about 6cm3 of distilled water and shake the mixture well. Divide the solution into 3 portions
| Observations | Inference |
| Dissolves to form uniform solution | polar solvent |
iii)To about 2cm3 of the solution, add a spatulaful of sodium hydrogen carbonate.
| Observations | Inference |
| effervecence/bobbles/fizzing | H+, R-CooH, H3O+ present |
iv)To about 2cm3, add 2 drops of acidified potassium manganate (vii) solution.
| Observations | Inference |
| purple H+KMnO4 decolourised | 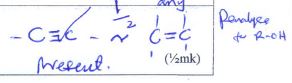 |
v)To another 2cm3, add 2 drops of bromine water.
| Observations | Inference |
| yellow bromine water decolourised |  |

CONFIDENTIAL
In addition to the apparatus and the fittings found in the chemistry laboratory, each candidate will require the following.
- One Burette, 0-50ml.
- One 25ml Pipette.
- Two 250ml Conical Flask
- One complete Retort Stand
- One White Tile
- One Pipette Filler
- Six Test-tubes
- Two Boiling tubes
- Wash bottle filled with distilled water
- One metallic spatula.
- About 1g of solid sodium hydrogen carbonate.
- Thermometer
- 120cm3 solution A.
- 120cm3solution B.
- Distilled water in a wash bottle.
- Solid R.
- Solid Q.
- Solid F.
ACCESS TO
- Source of heat.
- Acidified Potassium Manganite (VII)
- Bromine water
- Sodium sulphate solution
- Aqueous sodium hydroxide solution.
- Aqueous ammonia solution
- Acidified barium nitrate solution
NOTES
- Solid R is 2.0g Potassium chlorate, KClO3
- Solution A is prepared by dissolving exactly 39.2g of FeSO4(NH4)2SO4.nH2O in 600cm3 of distilled water and dilute with distilled water to one litre.
- Solution B is prepared by dissolving 3.0g of KMnO4 in 400ml of 2M H2SO4 and diluting with distilled water to one litre.
Download Chemistry Paper 3 Pre Mock Questions, Answers and Confidential - Mokasa I Joint Examination July 2021.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

