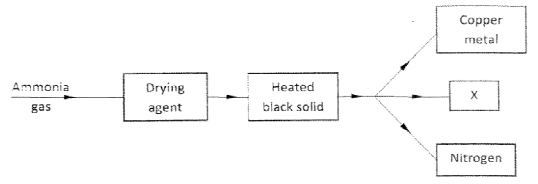
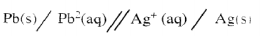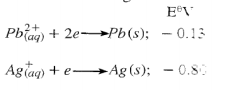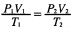- Charcoal is a fuel that is commonly used for cooking. When it burns it forms two oxides.
- Name the two oxides (2 marks)
- State one use of any of the two oxides (1 mark)
- Iron (III)oxide was found to be contaminated with copper (II) sulphate. Describe how a pure sample of iron (III) oxide can be obtained. (3 marks)
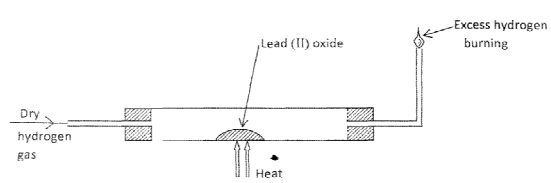
- In an experiment , dry hydrogen gas was passed over heated Lead (II) Oxide as shown in the diagram below.
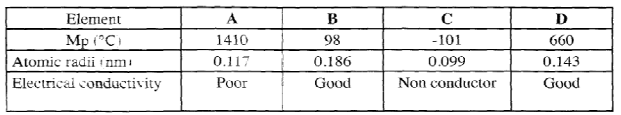
State the observations made in the combustion tube. (3 marks) - The table below shows properties of some elements A,B,C and D which belong to the same period of the periodic table . The letters are not the actual symbols of the elements.
- Arrange the elements in the order they woul appear in the period. Give a reason.(2 marks)
- Select the metallic element which is the better conductor of electricity. Give a reason ( 1 mark)
- A sample of water was found to boil at 101.50C at atmospheric pressure. Assuming that the thermometer was not faultye, explain this observation (1 mark)
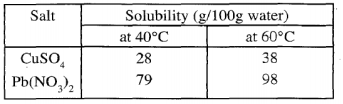
- Study the information in the table below and answer the questions that follow.
A mixture containing 35g of CuSO4 and 78g of Pb(NO3)2 in 100g of water at 600c was cooled at 400C- Which salt crystalised out? Give a reason (2 marks)
- Calculate the mass of the salt that crystallised out (1 mark)
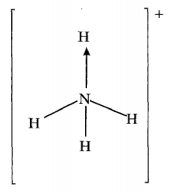
- Ammonium ion has the following structure
Label on the structure:- covalent bond; (1 mark)
- coordinate (dative) bond.(1 mark)
- 10 cm3 of concentrated sulpuric (VI) Acid was diluted in 100 cm3 of the resulting solution was neutralised by 36cm3 of 0.1M sodium hydroxide solution . Determine the mass of sulphuric (VI) that was in the concentrated acid (S=32.0;H=1.0;0=16.0) (3 marks)
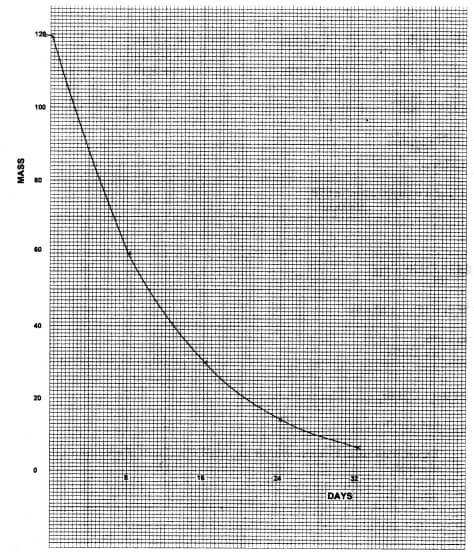
- 120g of iodine – 131 has a half life of 8 days and decays for 32 days . On the grid provided, plot a graph of the mass of iodine – 131 against time (3 marks)
- Name two cations that are present in hard water (1 mark)
- Explain how the ion exchange resin softens hard water (2 marks)
- The empirical formula of A is CH2Br . Given that 0.470g 0f A occupies a volume of 56cm3 at 546K and 1 atmospheric pressure, determine its molecular formular.(H=1.0. C=12.Br =80.0 molar mass volume at STP = 22.4 dm3) (3 marks)
- Study the flow chart below and answer the questions that follow.
- Name the suitable drying agent for ammonia. (1 mark)
- Describe one chemical test for ammonia.( 1 mark)
- Name x. (1 mark)
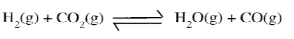
- A ytnamic equilibrium is established when hydrogen and carbom (IV) Oxide react as shown below.
What is the effect of adding powdered iron catalyst on the position of the equilibrium? Give a reason. (2 marks) - Distinguish between ionization energy and electron affinity of an element. (2 marks)
- Below is a representation of an electrochemical cell.
- What does // represent ? (1 mark)
- Given the following:
Calculate the E.M.F of the electrochemical cell. (2 marks)
- Use the following information on substances S,T,V and hydrogen to answer the questions that follow:
- T replaces V from a solution containing V ions.
- Hydrogen reacts with the heated oxide of S but has no effect on heated oxide of V.
- Arrange substances S, T , V and hydrogen in order of increasing reactivity. (2marks)
- If T and V are divalent metals, write an ionic equation for the reaction in (i) above . ( 1 mark)
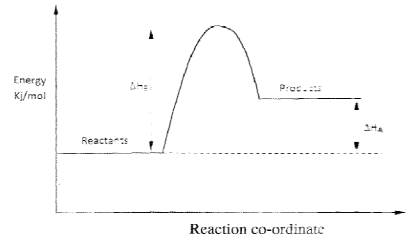
- Study the energy level diagram below and answer the questions that follow
- Give the name of ΔHA. (1 mark)
- How can ΔHb be reduced? Give a reason. ( 2 marks)
- Acidified potassium manganate (VII) solution is decoularisd when sulphur (IV) oxide IS bubbled through it. The equation for the reaction is given below.
2h20(l) + 5SO2(g) + 2KMnO4(aq) -----> K2SO4(aq) + 2H2SO4(aq)- Which reactant is oxidized ? Explain. (2 marks)
- Other than the manufacture of sulphuric (VI) acid state the other use of Sulphur (iv) oxide. (I mark)
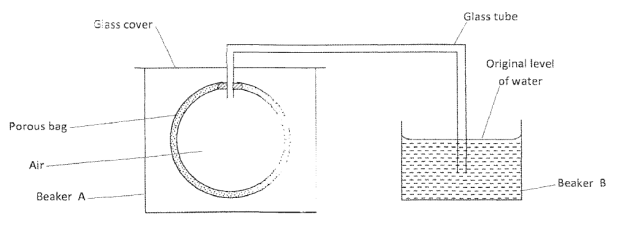
- The setup below was used to investigate a property of hydrogen gas.
State and explain the observation that would be made in the glass tube if beaker A was filled with hydrogen gas. ( 3 marks) - Draw and name the isomers of pentane. (3 marks)
- Give two uses of polymer polystyrene. ( 1 mark)
- Aluminium is both malleanle and ductile
- What is meant by?
- Malleable ( 1 mark)
- Ductible ( 1 mark)
- State one use of aluminium based on
- Malleability (1/2 mark)
- Ductility ( 1/2 mark)
- What is meant by?
- Describe how the percentage of mass of copper in copper carbonate can be determined. ( 3 marks)
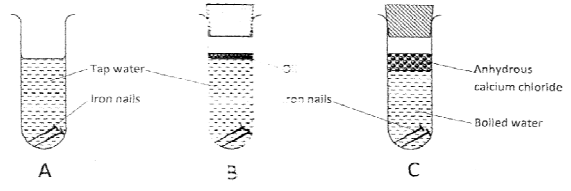
- The following set up of three tubes was used to investigate rusting of iron. Study it and answer the question that follow.
- Give a reason why rusting did not occur in test tube C. (1 mark)
- Aluminium is used to protect iron sheets from rusting. (2 marks)
Explain two ways in which aluminum protects iron from rusting. (2 marks)
- Describe how a solid sample of potassium sulphate starting with 200 cm3 of 2M potassium hydroxide. (3 marks)
- Describe two chemical tests that can be used to distinguish ethanoic acid. (3 marks)
- The electronic arrangement of ion of element Q is 2.8.8 . If the formula of the ion is Q3-, State the group and period to which Q belongs.
Group…………………….. (½ marks)
Period……………………..(½ marks) - Helium , neon and argon belong to group 8 of the periodic table . Give:
- The general name of these elements;( 1 marks)
- One use of these elements. (1 mark)
- The electronic arrangement of ion of element Q is 2.8.8 . If the formula of the ion is Q3-, State the group and period to which Q belongs.
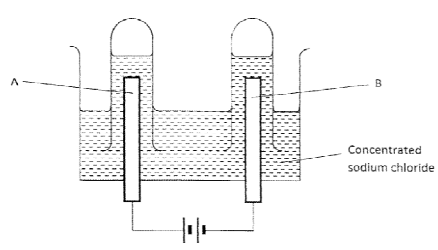
- The apparatus shown in the diagram below were used to investigate the products formed when concentrated sodium chloride was electrolysed using inert electrodes.
- Write the equation for the reaction that takes place at electrode A. ( 1 mark)
- If the concentrated sodium chloride was replaced with dilute sodium chloride , what product would be formed at electrode A? Explain. (2 marks)
-
- State and explain what would happen if a dry blue litmus paper was dropped in a gas jar of chlorine (1 mark)
- By using only dilute hydrochloric acid, describe how a student can distinguish between barium sulphite from barium sulphate.(2 marks)

MARKING SCHEME
-
- Carbon (IV) Oxide and Carbon (II) Oxide
-
- CO2
- Fire extinguishers
- Fizzy drinks
- Food preservative
- Solvay process
Choose 1
- CO
- Manufacture of fuel (water gas)
- Reduction in the extraction of metals
- Manufacture of methanol
Choose 1
- CO2
- Add water to dissolve CuSO4, while Fe2O3 does not. Filter out the undissolved Fe2O3, Wash residue with plenty of distilled water to remove traces of the filtrate. Dry the residue between filter papers.
- Grey Solid deposited PbO has been reduced to lead metal. A colourless liquid condenses on the cooler parts of the combustion tube. The hydrogen has been oxidised to water.
-
- BDAC
Across the period the atomic radius decreases. - D
Across the period the conductivity increase due to increase in delocalised electrons.
- BDAC
- The water must contain impurities. The presence of impurities elevates the boiling point.
-
- Copper (II) Sulphate; at 40°C only 28g is soluble leaving undissolved CuSO4, Pb (NO3) all dissolves.
- 35 - 28 = 7g
-
- H2SO4(aq) + 2NaOH(aq) → Na2SO4(aq) + H2O(l)
Moles of NaOH 36 × 0.1 = 0.0036
1000
Moles of acid in 100 cm3 = 100×0.0018 =0.018
10
R.M.M. of H2SO4 = 98(½)
0.0018 x 98 = 0.1764g(½) -
-
- Mg2+, Ca2+
- The Ca2+ and Mg2+ exchange with Nat on the ions exchange resin(1)
2R – Na + Ca2+ → R2 – Сa+Na+
2R – Na + Mg2+→ R2 – Mg + Na+
-
P2 = 1 litre P1 = 1 atm
V2 = ? V1 = 56cm3
T2 = 273K T1 = 546K
1 × V1 = 56 × 1
273 546
V1 = 56×1×273
546
V1 = 28cm3
0.47g of A occupies 28cm3 at STP
? 22400cm3
0.47 × 22400 = 376
28
CH2Br = 12 + 2 + 80 = 94
94n = 376
n=376/94
n = 4
(CH2Br)4 = C4H8Br4 -
- Calcium Oxide
- Expose ammonia to hydrogen chloride gas, dense white fumes of ammonium chloride are observed.
- Steam/water
- The catalyst has no effect on the position of equilibrium. The catalyst will increase the rate of forward and backward reactions to the same extent.
-
- Ionisation energy
This is the energy required to remove an electron from an atom in its gaseous state. - Electron affinity:
This is the energy change that results in the formation of an ion when an atom gains an electron
- Ionisation energy
-
- Represents salt bridge.
- EMF = EO gaining - EO losing
=+0.80 − (−0.13)
=+0.93V
-
- S, H, V,T (2) If only 1st and last letters are correct
- T(s) + V2+(aq) → T2+(aq) + V(s)
-
- Heat of reaction
- Using a catalyst
Catalyst reduce activation energy.
-
- Sulphur (IV) Oxide is oxidised
The change in Oxidation state for
SO2 → H2SO4
X = +4 → X = +6
Since it is increasing, this is oxidation. -
- Preservative for Jams and fruits
- Bleaching in the paper industry
- Fumigant
- Disinfectant
- Sulphur (IV) Oxide is oxidised
- The level of water in glass tube would go down this is because hydrogen gas being less dense than air diffuses through the porous bag, forcing the level of water in the glass tube to go down while the level of water in the beaker rises slightly.
- CH3 -CH2 - CH2 - CH2 - CH3 Pentane
2-methylbutane
2,2-dimethylpropane
-
- Plastic bottles
- Packaging of materials
- Tooth brush handles.
Any 1 (1)
-
-
- Malleable - Can be hammed into sheets
- Ductile - Can be drawn into wires
-
- Saucepans
- Electrical transmission lines
-
-
- Weigh copper carbonate
- Heat CuCO3 to constant mass in a combustion tube
- Reduce CuO using dry H2/NH3 or CO
- Allow to cool and reweigh to get mass of copper
- % = Mass of Cu × 100
Mass of CuCO3
-
- No air due to boiling.
-
- Aluminium being very reactive forms a layer of A1,0, on the metal making it impervious to moisture
- Aluminium being more reactive than iron protects the iron through sacrificial protection/ cathodic protection
- 2KOH(aq) + H2SO4(aq) → K2SO4(aq) + H2O.
Moles of KOH 200 x 2= 0.4 moles
1000
Moles of H2SO4 = 0.4 = 0.2 moles
2
x = 100cm3
Mix 200cm3 of 2M KOH with 100cm3 of 2M H2SO4 Concentrate the mixture to drive off excess water, crystallise using a water bath, then dry crystals between filter papers. -
- Add Na2CO3 or NaHCO3 to cach, with ethanoic acid there will be effervescence, no reaction with ethanol
- Add acidified potassium dichromate (VT) or acidified potassium manganate (VII) ethanol will decolarise acidified potasium manganate (VII) and change acidified potasium dichromate (VI) from orange to green. Ethanoic acid has no reaction with the reagent.
-
- Group is 5
Period is 3 -
- Noble gases/inert gases
- Used in fluorescence lamps, x-rays tubes
- Group is 5
-
- 2Cl−(aq) → Cl2(g) + 2e−
- Oxygen
There will be a higher concentration of the hydroxide ion in the dilute solution The hydroxide ion being higher in the electromotive series than the chloride ion will then be preferentially discharged.
-
- No change or no effect
Presence of water is necessary to form H+ and OCl−ions which change the litmus paper. - Add dilute hydrochloric acid to each of the salts. BaSO3 gives effervesence and the salt dissolves. there is no effervesence or effect on BaSO4
- No change or no effect
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download KCSE 2012 Chemistry Paper 1 with Marking Scheme.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students