- The grid given below represents part of the periodic table. Study it and answer the questions that follow. The letters do not represent the actual symbols of the element.

- Select a letter which represents an element that loses electrons more readily
Give a reason for your answer. (2 marks) - Explain why the atomic radius of P is found to be smaller than that of N. (2 marks)
- Element M reacts with water at room temperature to produce 0.02 dm3 of gas. Determine the mass of M which was reacted with water. (Molar gas volume at room temperature is 24 dm3, Relative atomic mass of M = 7)
- Select a letter which represents an element that loses electrons more readily
- Use the information in the table below to answer the questions that follow.
(These letters are not the symbols of the elements.)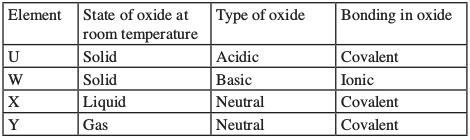
Identify a letter which represents an element in the table calcium, carbon or sulphur. Give a reason in each case.
- The grid given below represents part of the periodic table. Study it and answer the questions that follow. The letters do not represent the actual symbols of the element.
-
- What is meant by the term 'Enthalpy of formation'? (1 mark)
- The following enthalpies of combustion of carbon, methane and hydrogen are indicated below:
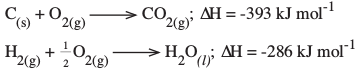
Enthalpy of combustion of CH4 = -890 kJ mol-1- Draw an energy cycle diagram that links the enthalpy of formation of methane to enthalpies of combustion of carbon, hydrogen and methane. (2 marks)
- Determine the enthalpy of formation of methane. (2 marks)
- An experiment was carried out where different volumes of dilute hydrochloric acid and aqueous sodium hydroxide both at 250C were mixed and stirred with a thermometer. The highest temperature reached by each mixture was recorded in the table below:
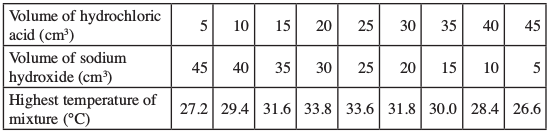
- On the grid provided, plot a graph of hoghest temperature (vertical axis), against volume of hydrochloric acid (horizontal axis). (3 marks)
- Using your graph, determine the:
- Highest temperature reached; (1/2 mark)
- Volume of acid and base reacting when the highest temperature is reached; ( 1/2 mark)
- Calculate the amount of heat liberated during the neutralisation process.
(Specific heat capacity is 4.2 g-1K-1 and the density of the solution is 1.0 g cm-3) (2 marks)
- On the grid provided, plot a graph of hoghest temperature (vertical axis), against volume of hydrochloric acid (horizontal axis). (3 marks)
- The molar neutralisation between hydrochloric acid and ammonia solution was found to be -52.2 kJ mol-1, while that of hydrochloric acid and sodium hydroxide was -57. 1 kJ mol-1. Explain the difference in these values. (2 marks)
-
- The diagram below shows the Frasch process used for extraction of sulphur.
Use it to answer the questions that follow.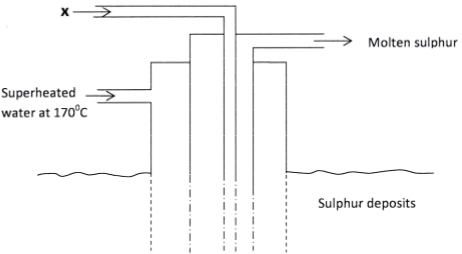
- Identify X. (1 mark)
- Why is it necessary to use super heated water in this process? (1 mark)
- State two physical properties of sulphur that makes it possible for it to be extracted by this method. (2 marks)
- The diagram below shows part of the processes of manufacture of sulphuric (VI) acid. Study it and answer the questions that follow.
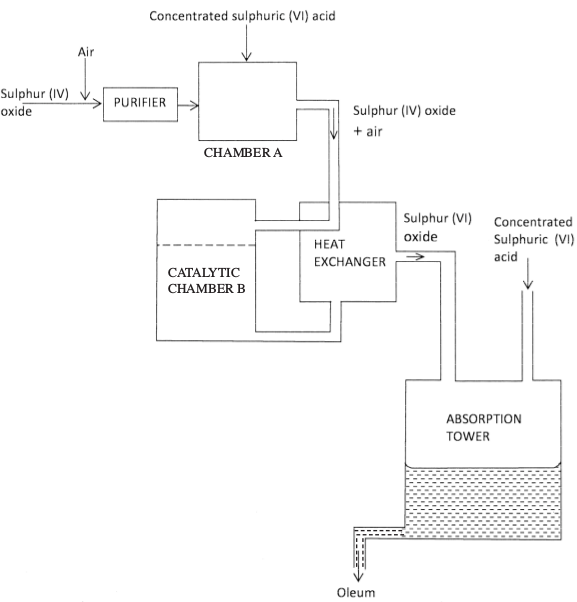
- Write an equation for the formation of sulphur (IV) oxide from sulphur. (1 mark)
- What is the role of concentrated sulphuric (VI) acid in chamber A? (1 mark)
- Name two catalysts that can be used in catalystic chamber B. (2 marks)
- State two roles of heat exchanger. (2 marks)
- Explain one way in which sulphur (IV) oxide is a pollutant.
- What observations would be made when a few drops of concentrated sulphur (VI) acid are added to crystals of sugar? Explain your answer. (1 mark)
- The diagram below shows the Frasch process used for extraction of sulphur.
-
- The set up below can be used to produce sodium hydroxide by electrolysing brine.
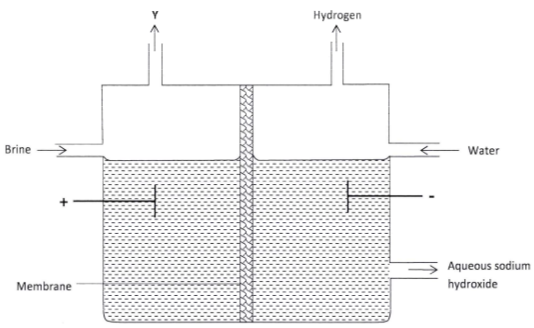
- Identify the gas Y. (1 mark)
- Describe how aqueous sodium hydroxide is formed in the above set-up. (2 marks)
- One of the uses of sodium hydroxide is in the manufacturing of soaps.
State one other use of sodium hydroxide. (1 mark)
- Study the information given in the table below and answer questions that follow.
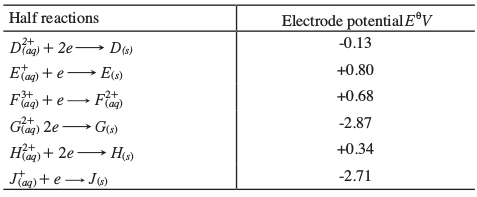
- Construct an electrochemical cell that will produce the largest emf. (3 marks)
- Calculate the emf of the cell constructed in (i) above. (2 marks)
- Why is it not advisable to store a solution containing E+ ions in acontainer made of H? (2 marks)
- The set up below can be used to produce sodium hydroxide by electrolysing brine.
-
- Describe one method that can be used to distinguish between sodium sulphate and sodium hydrogen sulphate. (2 marks)
- Describe how a pure sample of lead (II) sulphate can be prepared in the laboratory starting with lead metal. (3 marks)
- Study the flow chart below and answer the questions that follow.
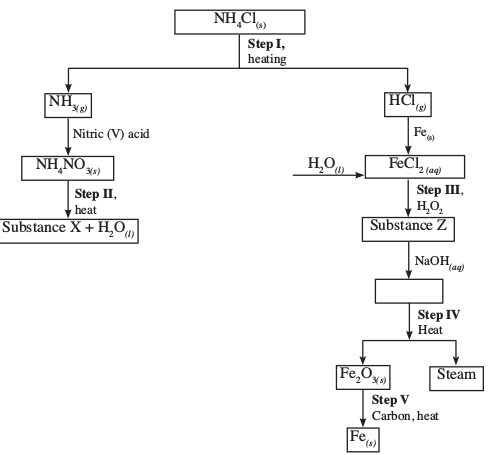
- Write the equation for the reaction in:
- Step II (1 mark)
- Step IV (1 mark)
- State the observations made in step III. Explain. (2 marks)
- Name another substance that be used in step V. (1 mark)
- Write the equation for the reaction in:
-
- Distinguish between a neutron and a proton. (1 mark)
- What is meant by radioactive substance? (1 mark)
- State two dangers associated with radioactive substances in the environment. (2 marks)
- The isotopes of hydrogen , deuterium (21D) and tritium (31T) react to form element Y and neutron particles, according to the equation below:
21D + 31T -----> abY + 10n- What is the atomic:
- mass of Y; (1 mark)
- number of Y. (1 mark)
- What is tha name given to the type of reaction undergone by the isotopes of hydrogen? (1 mark)
- What is the atomic:
-
- What is meant by the half-life of a radioactive substance? (1 mark)
- 288g of a radioactive substance decayed to 9g in 40 days. Determine the half-life of the radioactive substance. (2 marks)
-
- Give the systematic names for the follwing compounds:
- CH3CH2COOH; (1 mark)
- CH3CH2CH2CHCH2 (1 mark)
- CH C CH2CH3. (1 mark)
- Study the flow chart below and use it to answer the questions that follow:
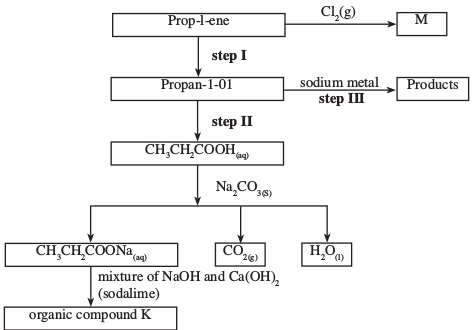
- Identify the organic compound K. (1 mark)
- Write the formula of M. (1 mark)
- Give one reagent that can be used in:
- Step I; (1 mark)
- Step II. (1 mark)
- Write an equation of the reaction in step III. (1 mark)
- The structure below represents a type of a cleansing agent.
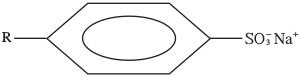
Describe how the cleansing agent removes grease from a piece of cloth. (3 marks)
- Give the systematic names for the follwing compounds:

MARKING SCHEME
-
-
- R - (1) it has the largest atomic radius with the weakest nuclear attraction for outermost electron (1).
- Across the period the atomic radius decreases due to the increase in nuclear attraction (1). Number of electrons in P is greater than in H.
M(s) + H2O(l) → 2MOH(aq) +H2(g) (1) - Moles of H2 = = 0.0083
Moles of M = 0.0083 x 2 = 0.0166
Moles of M = 0.0166
RAM
Mass of M = 0.0166 x 7
Mass of M = 0.117 g
-
- W - (1) forms a basic oxide which forms an ionic bond (1).
- Y - (1) the oxide is gaseous that forms a neutral solution (1).
- U - (1) the oxide is solid at room temperature, which is acidic with covalent bond (1).
-
-
-
- This is the heat absorbed or evolved when one mole of any substance is formed from its constituent elements in their normal states. (1 mark)
-
-
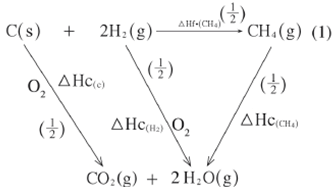
- 3 Hf^CH4h = 3 Hc^ch + 2 3 Hc^H2h - 3 Hc^CH4h
= - 393 + 2^- 286h + 890 (1)
= - 965 + 890
= - 75 kJ mol-1 (1)
-
-
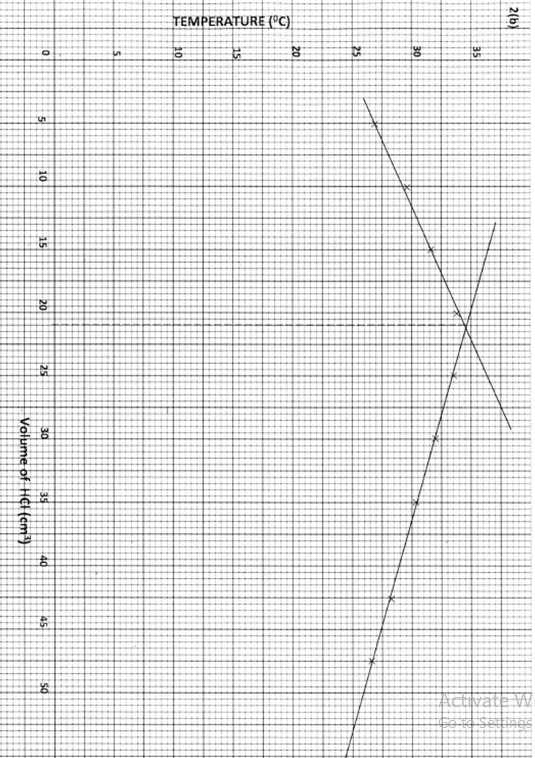
(3 marks)-
- 34.8℃
- 21.2 cm3 HCl
- 50 x 9.8 x 4.2
= 2058 Joules (1)
- The molar heat of neutralisation between a strong acid and a weak base is low because some of the heat is used to ionise (1) the weak base before neutralization.
For strong acid and strong base they are completely ionised.
-
-
-
- Hot compressed air (1)
- To melt the sulphur and maintain it in molten state (1)
-
- low melting point of sulphur (1)
- insolubility of sulphur in water (1)
- less dense than water
-
- S^sh + O2^gh $ SO2^gh (1)
- To dry the SO2 and air (1)
- Vanadium (v) oxide (1) and platinum (1) or titanium
-
- it provides the reactants (SO2 and O2) with enough energy to react (1)
- it removes heat from the product hence preventing decomposition (1) or conserves heat, or recycles heat or reduces cost of production.
Accept any other.
-
- contributes to acid rain which corrodes buildings (1)
OR - causes aquatic solutions to be acidic hence affecting aquatic life etc.
- poisonous/toxic
- contributes to acid rain which corrodes buildings (1)
- Turns black conc H2SO4 removes hydrogen and oxygen from the sugar molecule leaving only carbon which is black. Dehydration of sugar forms carbon which is black.
-
-
-
- Gas Y is chlorine. (1)
-
- sodium and hydrogen ions migrate to the cathode The hydrogen ions are preferentially discharged, liberating hydrogen gas.
- chlorine and hydroxide ions migrate to the anode The chloride ions are preferentially discharged liberating chlorine gas.
- the sodium ions migrate to the cathode through the membrane
- the sodium ions combine with the hydroxide ions to form sodium hydroxide
- Glass making/paper manufacture (1), unclogging of drains, etching NaClo3, Purification of bauxite.
-
-
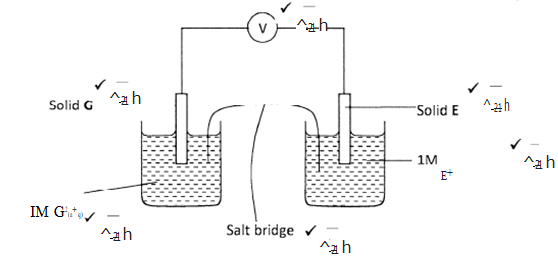
- EMF = 0.8 + 2.87 (1)
= 3.67V (1) - H will go into solution as H2+ ions (1) since it is more reactive than E hence displacing E+ ions which are deposited as solid (1).
-
-
-
- Test the acidity using a litmus pager. There will be no change on litmus when dipped into a solution of sodium sulphate (1). The litmus paper turns to red when dipped into a solution of sodium hydrogen sulphate (I).
OR
Add a solid carbonate to each solution. No effervescence observed when the carbonate is added to a solution of sodium sulphate. Effervescence is observed when the carbonate is added to a solution of sodium hydrogen sulphate. - Add dilute nitric acid to lead to form a soluble salt, Pb(NO3)2, add a soluble salt sodium sulphate to form insoluble, PbSO4 and soluble Na2SO4 separate by filtrating.Wash the PbSO4 with distilled water to remove traces of soluble salt, Na2SO4. Then dry the salt between filter papers
-
-
- NH4 NO3^sh $ N2 O^gh + 2H2 O^gh (1)
- 2Fe(OH)3(S) Fe2O3(s) + 3H2O(l)
- The colour changes from pale green to brown (1) . The iron (II) is oxidised to iron (III) chloride by hydrogen peroxide (1)
- Carbon monoxide (1)
-
- Test the acidity using a litmus pager. There will be no change on litmus when dipped into a solution of sodium sulphate (1). The litmus paper turns to red when dipped into a solution of sodium hydrogen sulphate (I).
-
- A proton has a +ve charge while a neutron has no charge (1)
- Substances undergo radioactive decay or disintergration. (1)
-
- causes genetic mutation (1)
- can cause death (1)
- prone to cancer
-
-
- Atomic mass of a = 4 (1)
- Atomic number of b = 2 (1)
- Fusion (1)
-
-
- This is the time taken for half of the radioactive isotope to decay (1)
- 288 144 72 36 18 9
`5 half lives (1)
40 = 8 days (1)
5
-
-
- Propanoic acid (1)
- Pent - l - ene (1)
- But - 1 - yne (1)
-
- Ethane (1)
- C3H6Cl2 (1)
-
- Water/steam/Conc. H2SO4 (1)
- Acidified potassium dichromate (VI)
- 2CH3 CH2 CH2 OH + 2Na 2CH3 CH2 ONa + H2
- Cleansing agent has the hydrophilic and hydrophobic ends, the hydrophobic end is attracted to grease while the hydrophilic end is attracted to water during agitation the grease is pulled off ^21 h the cloth then surrounded by soap molecules
-
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Kenya Certificate Of Secondary Education(KCSE 2013) Chemistry Paper 2 with Marking Scheme.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students