Instructions to Candidates
- Write your name, Admission number and index number in the spaces provided
- Sign and write the date of examination in the spaces provided above
- Answer ALL the questions in the spaces provided below each question.
- Mathematical tables and silent electronic calculators may be used.
- All working MUST be clearly shown where necessary.
- This paper consists of 13 printed pages
- One of the basic laboratory rules is that before using the reagents, it is important to read the labels on the reagent bottles.state the reason for this. (1mrk)
-
- Describe how oil can be extracted from the sunflower seeds. (2marks)
- Two physical states of matter can be represented as shown below in the diagram below.
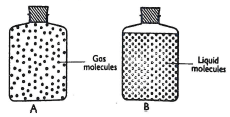
- State the observation that shows that the liquid particles do not move freely (Imrk)
- Explain why liquid molecules are closer to each other than gas molecules (Imrk)
-
- Sulphur (IV) Oxide has many uses complete the table below by indicating the role played by sulphur (IV) Oxide in the following.(2mrks)
Area of Use Role of sulphur(IV) oxide Grain silos Fruit and food processing - Sulphuric acid is used in metallurgical industries to clean steel before it is galvanized. Explain the role of sulphuric acid. (1mrk)
- The figure below is for dissolving Chlorine gas was bubbled potassium bromide. Study it and answer the questions that follow.
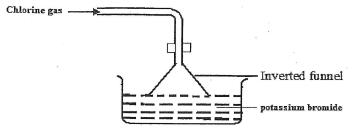
State and Explain the observation made in beaker. (2mrks) - The structures below are a representation of cleansing agents M and N.
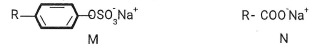
- Identify the agents. (1 mark)
M................N................. 11) - Explain why agent M causes water pollution. (Imrk)
- Tetraoxophosphate is usually added to remove Cap and Mgo during cleaning and provides nutrients for aquatic plants and they grow quickly depleting the dissolved oxygen, Name the process described. (1mrk)
- Identify the agents. (1 mark)
- Calculate the percentage abundance of two other isotopes of element A with three isotopes, (30,32, and 35). Given that the R.A.M is 30.5 and percentage abundance of 35 is 5%. (2 marks)
- Phosphorus (III)oxide has a lower melting point than calcium chloride. Explain. (2mrks)
- Use the table below to determine the Bond energy for CI-CI (2 marks) Given H2(g) + Cl2(g)- 2HCI(g) H=-184kj/mole
Bond H-H H - CI Bond Energy (kJ) 435 431 -
- Name the major raw material from which Aluminium is extracted. (Imrk)
- Explain why cryolite is added to aluminium oxide before electrolysis. (1 mark)
- Explain why Aluminium is extracted using electrolysis. (1 mrk)
-
- Define the term half-life. (1 mark)
- A certain nuclide has a half-life of 2.5 hours. What percentage of a given mass of the nuclide will be left after 7.5 hrs? (2 marks)
-
- Explain how increase in Temperature affects the rate of a given reaction. (2 marks)
- In the Haber process, the industrial manufacture of ammonia is given by the following equation;
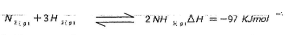
- Name the catalyst used in the above reaction(1mk)
- What is the effect of increasing temperature on yield of ammonia? Explain. (1mk)
- The following diagrams show the structure of two allotropes of carbon. Study them and answer the questions that follow.
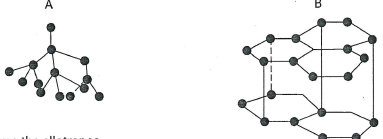
- Name the allotropes (1 mark)
A..................B........................ - Which allotrope conducts electricity? Explain.(2 marks)
- Name the allotropes (1 mark)
- 20cm3 of 2M sulphuric acid was reacted completely with 3.2g of ROH (0=16, H=1) Calculate the R.A.M of R in the formula ROH.(2mrks)
- An organic compound with the formula C4H10O reacts with sodium metal to give Hydrogen gas and white solid.
- To which homologous series does white solid belong? (1mrk)
- Write the equation for the reaction between the organic compound C4H10O and sodium metal. (1mrk)
- Fluorine, Chlorine and Bromine has the boiling point of -188,-35 and 59 Explain the trend in the boiling points. (2mrks)
- Electroplating refers to the process of coating a reactive metal with a layer of less reactive.
- Give ONE reason for electroplating (1mrk)
- During Electroplating of iron spoon with copper the type of electrolyte that should be used is copper (ii) sulphate solution. Write the equation for the reaction at the cathode. (1mrk)
- The table below gives the solubilities of Sodium Bromine and Sodium Sulphate at 20°C and at 60°C
When aqueous mixture containing 60g of NaBr and 7g of Na2SO4 in 100g water at 80°C was cooled to 20°C, some crystals were formed;Substance Solubility /100g water 20°C 60°C Sodium Bromide 55 75 Sodium Sulphate 10 12 - Identify the crystals (1mk)
- Determine the mass of the crystals formed (1mk)
- Name the method used to obtain the crystals (1mk)
- Given below are molar enthalpies of combustion of propane, hydrogen and carbon.
C3H8(g)+ 5 O2(g) → 3CO2(g)+ 4H2O(I); ΔH = - 2220 kJ/mole
H2(g) + ½ O2(g) → H2O(g) ΔH = -286kJ/mole
C(s) + O2(g) → CO2(g) ΔH =-393 Kj/mole
Use the above information to calculate the molar enthalpy of formation of propane. (3mks) - Magnesium is hard to cut with a knife however it is Ductile and Malleable while calcium is brittle hence cannot be cut with a knife. Define.
- Ductile. (1mrk)
- Some meals produces sound when strucked, Name the property described. (1mrk)
- Some metals were reacted with cold water; a hydrogen gas is given off. The diagram below shows reactions of the metals.
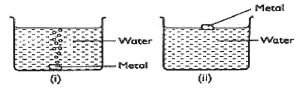
- Predict the metals in set-up () and (ii) (1mrk)
- Write equation for the reaction taking place in set-up 0) (1mrk)
- When manure rots in tanks called digester, biogas which is a mixture of 60% methane and 40% carbon (IV) Oxide is formed.Biogas is used as a fuel.
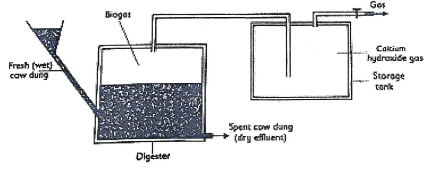
- Explain how calcium hydroxide in the gas storage improve the fuel.(1mrk)
- State one disadvantage of using biogas as a fuel. (1mrk)
- Give ONE use of the Dry effluent. (1mrk)
- Electron arrangement in an atom of nitrogen and in atom hydrogen are shown.
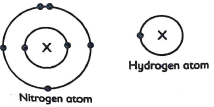
- Explain why the atom of hydrogen smaller than that of Nitrogen (Imrk)
- Name the region of the atoms shown by X.(1mrk)
- Draw structure of the molecule formed when the two elements reacts. (1mrk)
- Chlorine can be prepared by Oxidation of chloride ions by oxidizing agents as shown in the diagram below.
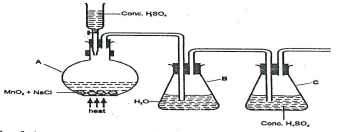
Explain the function of the following.- Concentrated sulphuric(VI) Acid in the reaction vessel A. (1mrk)
- Water in container B.(1mrk)
- Complete the set-up to show how dry chlorine gas is collected. (1mrk)
- What observation would be made on the blue litmus paper placed in a solution of hydrogen chloride in water? Explain(2mks)
-
- Give the name of the third member of the alkyne homologous series (1mrk)
- Describe chemical test to distinguish propane and Prop-1-yne. (2mrks)
-
- State Boyles law. (1 mrk)
- A gas occupies 4dm3. at a pressure of 152mmHg. Calculate the gas pressure when the volumes are reduced to 1.5dm3. (2mrks)
-
- Name two anions which caused permanent hardness in water. (1mrk)
- Describe how sodium carbonate can be used to remove water hardness. (2mrks)
- The set-up was used in an experiment to show the reaction of Magnesium with Nitrogen, Drops of water were added after the Experiment.
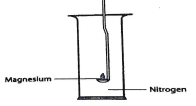
- What would be observed when moist red litmus papers is placed at the mouth of the jar after experiment. Explain. (1mrk)
- State and Explain the observation that would be made when burning sulphur is used instead magnesium.(2mrks)
- A burning candle was covered with beakers of different sizes. The time taken for the candle to go off in the different beakers was recorded.
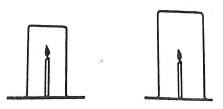
- Explain why the same kind of candles be used in all the experiments.(1mrk)
- Explain why the candle takes longer to go off in the larger beaker (1mrk)
- The experiment was repeated with a shorter candle. The time recorded for the candle to go off was longer than in the first experiment.
- Explain the observation. (Imrk)
- Carbon (IV) Oxide and water Vapour are some of the greenhouse gases. 1)Explain how greenhouse gases contributes to global warming. (1mrk)
- State the steps taken to mitigate the effects of global warming (1mrk)
- The diagram below shows the distribution of air molecules and water molecules in a closed container at a certain temperature.
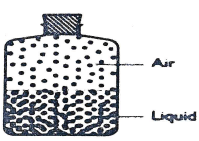
- Describe the approximate distribution of air and liquid molecules in the container at a higher temperature.(1mrk)
- Identify the physical property of the particles in the container that has.
- Increased. (1mrk)
- Decreased. (1mrk)
-
- Name tive family to which group Il elements belong. (1mrk)
- Identify one element which belong to the family above and is in period 4. (1mrk)
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Questions - Alliance Boys High School Post Mock KCSE 2020.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

