QUESTIONS
- The grid below represents part of the periodic table. Study it and use it to answer the questions that follow. The letters do not represent actual symbols of the elements.
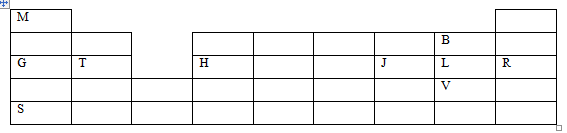
- An element X forms a divalent cation with the electron configuration 2.8.8. Place element X in its position on the grid (1 mark)
- Element G was put in a trough with cold water containing phenolphthalein indicator
- State two observations made during the reaction (2 marks)
- Write a chemical equation for the reaction (1 mark)
- Compare the reactivity of G and S with cold water. Explain (2 marks)
- Draw dot(o) and cross (x) diagram showing bonding when element T and element L combine to form a compound. (1 mark)
- Kamau accidentally mixed a chloride of S, iron (III) chloride and an oxide of H. Describe how he obtained a solid sample of each. (3 marks)
- Explain why at room temperature, an oxide of G is a solid while an oxide of J is gaseous. (1 mark)
- State one use of element R (1 mark)
- The flow diagram below shows reactions starting with propanol. Study it and use it to answer the questions that follow.
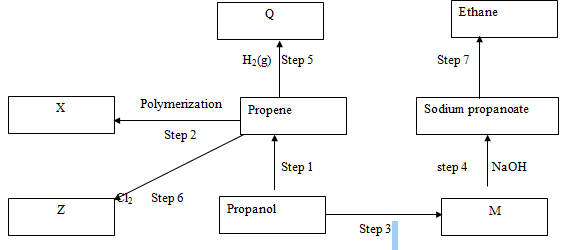
- Name the process in (1 mark)
- Step 1 ………………………………………………………………………………
- Step 3 ………………………………………………………………………………
- State the condition in (2 marks)
- Step 1 ………………………………………………………………………………
- Step 5 ………………………………………………………………………………
- Draw the structure of substance (2 marks)
- X
- Z
- Name the reagent used in (2 marks)
- Step 7 ………………………………………………………………………………
- Step 3 ………………………………………………………………………………
- Identify substance (1 mark)
- M ………………………………………………………………………………
- Q ………………………………………………………………………………
- Describe an experiment used to distinguish between the product in step 1 and step 7 (2 marks)
- Write an equation for the reaction of
- Propanol with potassium (1 mark)
- Propene with oxygen (1 mark)
- Name the process in (1 mark)
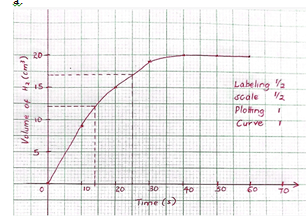
- A student reacted 6g of magnesium ribbon with 50 cm3 of 0.1M Hydrochloric acid and measured volume of hydrogen gas given off every 10 seconds for 60 seconds. The table below gives the results obtained.
Volume of hydrogen gas (cm3) 0 9 15 19 20 20 20 Time taken (seconds) 0 10 20 30 40 50 60 - On the grid below, plot a graph of Volume of hydrogen gas (y – axis) against time (x – axis) (3 marks)
- From the graph determine:
- Volume of gas produced at time 25 seconds ………………………………. (1 mark)
- Time taken for 12 cm3 of hydrogen gas produced …………………………… (1 mark)
- Explain the shape of the curve between 40 – 60 seconds (1 mark)
- The experiment was repeated using 1M Hydrochloric acid.
- On the same axes sketch the curve that would be obtained (1 mark)
- Explain your answer in d(i) above (1 mark)
- Study the flow diagram below and use it to answer the questions that follow
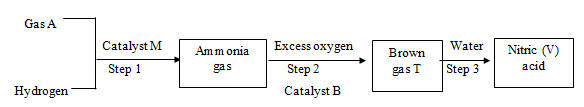
- Name; (2 marks)
- Gas A ………………………………………………………………………………
- Catalyst M ………………………………………………………………………………
- Catalyst B ………………………………………………………………………………
- Gas T ………………………………………………………………………………
- Write an equation for: (2 marks)
- Step 1
- Step 3
- Name the main source of gas A (1 mark)
- Ammonia gas was passed through a combustion tube containing heated copper (II) oxide as shown in the diagram below.
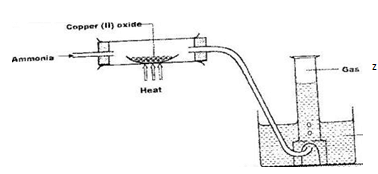
- State and explain one observation made in the combustion tube (2 marks)
Identify gas Z (1 mark) - What property of ammonia is being investigated? (1 mark)
- Name a suitable drying agent for ammonia gas (1 mark)
- State and explain one observation made in the combustion tube (2 marks)
- Name; (2 marks)
- The diagram below shows the set – up used to investigate enthalpy of combustion of ethanol when 450cm3 of water was heated
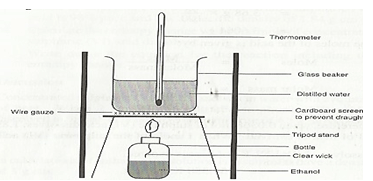
The data below was obtained during the experiment
Volume of water = 450 cm3
Initial temperature of water = 23.0 ºC
Final temperature of water = 41.0 ºC
Mass of the lamp + ethanol before heating = 141.7g
Mass of the lamp + ethanol after heating =140.2 g
Density of water = 1g/cm3
Specific heat capacity = 4.2 Kj Kg-1 K-1- Calculate;
- Heat evolved during the experiment (2 marks)
- Moles of ethanol that reacted (C=12.0, H= 1.0, O=16.0) (1 mark)
- Molar heat of combustion of ethanol (2 marks)
- Write a thermochemical equation for the reaction (1 mark)
- The theoretical molar enthalpy of combustion of ethanol is – 1260 kJ/Mol. Give two reasons why the experimental value is less (2 marks)
- Name two factors to consider before choosing a fuel (2 marks)
- Study the information below and use it to answer the questions that follow
∆Hθ lattice = MgCl2 - 2477kjmol-1
∆Hθ hydration Cl-1 (aq) -363kjmol-1
∆Hθ hydration Mg+2 (aq) -1891jmol-1- Define the molar enthalpy of solution combustion of a substance? (1 mark)
- Using the above information Draw an energy level diagram to represent the heat of solution of Magnesium Chloride (1 mark)
- Calculate the heat of solution of Magnesium Chloride (2 marks)
- Calculate;
- Use the reduction potentials below for P, Q, R, S and T to answer the questions that follow.
Reaction Eº value (V) P2+ (aq) + 2e- → P (s) -0.79 2Q+ (aq) + 2e- → Q2 (s) 0.00 R2+ (aq) + 2e- → R (s) +0.45 S2+ (aq) + 2e- → S (s) -0.21 ½ T2 (g) + 2e- → T - (aq) +2.91 - Identify;
- The element that is likely to be hydrogen (1 mark)
- The strongest reducing agent (1 mark)
- The half cells of P and R were combined
- Draw the electrochemical cell formed (3 marks)
- Calculate the e.m.f. of the cell formed (1 mark)
- During the extraction of sodium using the Down’s cell, molten sodium chloride is electrolyzed.
- State the role of the following in the cell (2 marks)
Calcium chloride
Steel diaphragm - State the observation made at the anode (1 mark)
- Write an equation for the reaction at the cathode (1 mark)
- 2A was passed through molten sodium chloride for 2 hours and 35 minutes. Calculate the mass of sodium metal formed (1F= 96,500C, Na=23, Cl=35.5) (2 marks)
- State the role of the following in the cell (2 marks)
- State two applications of electrolysis (2 marks)
- Identify;
- The set up below was used to prepare hydrogen chloride gas and salt T.
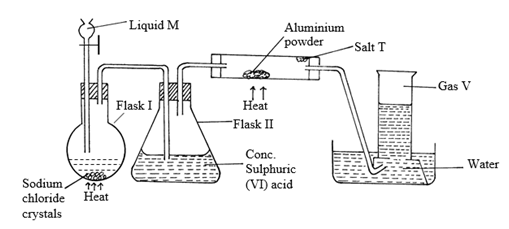
- Identify the following
- Liquid M……………………………………………………………………. (1 mark)
- Gas V………………………………………………………………………. (1 mark)
- Salt T……………………………………………………………………… (1 mark)
- Write balanced chemical equations for reactions that occur at:
- Flask I (1 mark)
- Combustion tube. (1 mark)
- Name the process that formed salt T as shown in the diagram. (1 mark)
- Sulphuric (VI) acid is used as a drying agent in this experiment. Explain why calcium oxide is unsuitable for the same purpose in this reaction. (1 mark)
- The water in the trough was found to have a pH of 2.0 at the end of the experiment. Explain. (1 mark)
- In the space provided below, draw a well labelled diagram showing how you would dissolve hydrogen chloride gas in water. (1 mark)
- Explain why hydrogen chloride gas dissolved in methylbenzene does not react with calcium carbonate. (1 mark)
- Identify the following

MARKING SCHEME
-
- Group II, period 4
-
- Darts on the surface
Floats on water
Hissing sound
Indicator changed from colourless to pink (any 2 correct) - 2G(s) +2H2O(l) →2GOH(aq)+H2(g)
- S is more reactive. It has more energy levels hence loses an electron more readily
- Darts on the surface
-
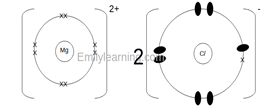
- Heat the mixture to sublime iron (III) chloride. ½ Add water to the remaining mixture to dissolve the chloride of S ½ Filter to obtain the chloride of S as filtrate and oxide of H as residue ½ heat the filtrate to obtain a saturated solution ½ Allow the saturated solution to cool ½ Wash the residue and dry ½
- An oxide of G has giant ionic structure ½ with strong ionic bonds ½ hence a solid. An oxide of J has molecular structure ½ with weak van der waal’s forces
- Provide an inert atmosphere during welding
Used in filament bulbs
-
-
- Dehydration
- Oxidation
-
- X
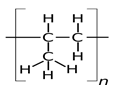
- Z
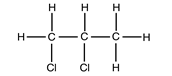
- X
-
- 170 – 180ºC
- Nickel catalyst and 150ºC MENTION BOTH
-
- Sodalime
- Acidified potassium manganate (VII) or potassium dichromate (VI)
- M – Propanoic acid
Q – Propane
OR STRUCTURAL FORMULAE - Pass the products separately through acidified potassium manganate (VII). Using Ethane the solution remains purple. Using propene the solution changes from purple to colourless.
-
- 2CH3CH2CH2OH+2K→2CH3CH2CH2OK+H2
- 2CH3CHCH2 + 9O2→ 6CO2 + 6H2O
-
-
-
-
- 17+- 1cm3
- 14+-1 s
- The curve is constant as one or both reactants is completely used up.
-
- curve flattens at time < 40seconds
- 1M HCl has a higher concentration hence reaction is faster and takes a shorter time
-
-
-
- Nitrogen
- Finely divided iron
- platinum – rhodium
- Nitrogen (IV) oxide
½ks each
-
- N2(g) + 3H2(g) → 2NH3(g)
- 4NO2(g) + 2H2O(l) + O2(g)→ 4HNO3(aq)
or NO2(g) + H2O(l) → HNO3(aq) +HNO2(g)
- fractional distillation of air
-
- solid changes from black to brown as copper (II) oxide is reduced to copper
- nitrogen
- reducing property
- calcium oxide
-
-
-
- 450/1000 x 4.2 x 18 = 34.02kJ
- 1.5 / 46 = 0.03261 moles
- 1 x 34.02/0.03261 = –1043.28kJ/Mol
penalize ½ for missing sign
- CH3CH2OH + 5O2 → 3CO2 + 4H2O ΔH = -1043.28Kj/Mol
- Some heat is lost to the surrounding / heat absorbed by apparatus
Incorrect reading of values
Incomplete combustion of ethanol - cost, transport, heating value, storage, effect to environment
-
- This is the enthalpy change when one mole of a substance dissolves in a solvent to form an infinitely diute solution .
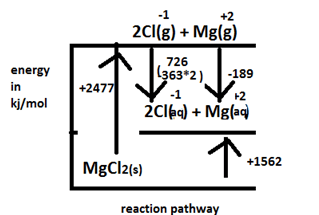
- 4 (- 399kjmol-1) + 5 (- 286kjmol-1) = ∆Hf + - 2877kjmol-1
2477 +(-363*2) +(-189) = ∆Hsol
∆Hsol = +1562kJ/mol
- This is the enthalpy change when one mole of a substance dissolves in a solvent to form an infinitely diute solution .
-
-
-
- Q
- P
-
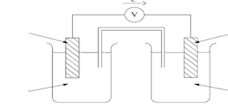
½ FOR EACH ARROW AND SALT BRIDGE- 0.45 – (- 0.79) = + 1.24V
-
- calcium chloride- lowers melting point of the ore from 800-600
steel diaphragm – prevents chlorine and sodium from recombining - green gas
- Na+ (l) + e → Na(l)
- time = (2 x 3600) + (35 x 60) =9300
Q = 2 x 9300 = 18,60ºC
Na+ (l) + e → Na
Mass = (18600x23)/96500 = 4.4332g
- calcium chloride- lowers melting point of the ore from 800-600
- Extraction of reactive metals
Electroplating metals to prevent rusting and improve appearance
Purification of metals
manufacture of pure chemicals like Chlorine, hydrogen gas, sodium hydroxide
-
-
-
- Conc. Sulphuric (VI) acid /sulphuric acid √ Acc. formula
- Hydrogen gas // H2 √
- Aluminium Chloride // AlCl3 √
-
- NaCl(s) + H2SO4(l) → Na2SO4(aq) + 2HCl(g) √ or Deny ½ if state symbols wrong or missing
H2SO4(l) + Cl-(aq) → H2SO4(aq) + HCl(g) - 2Al(s) + 6HCl(g) → 2AlCl3(s) + 3H2(g) √
- NaCl(s) + H2SO4(l) → Na2SO4(aq) + 2HCl(g) √ or Deny ½ if state symbols wrong or missing
- Displacement √
- Gas is acidic, therefore reacts with the basic calcium oxide √
- Unreacted HCl gas √ dissolves forming a strongly acidic solution √ Accept correct equation
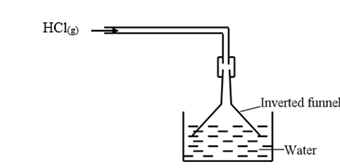
workability = √½, Labelling = √½- In methyl / benzene HCl exists in molecular form and there are no H+ ions to react with the carbonate √½
-
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 2 Questions and Answers - MECS Cluster Joint Mock Exam.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students
