3.5 CHEMISTRY (233)
In 2017, Chemistry, 233 was tested using two theory papers and a practical paper. 233/1 and 2 are theory while 233/3 is a practical paper which tests on practical skills attained by candidates.
The table below shows performance of chemistry (233) for the last 5 years.
Table 12: Performance of Chemistry in the Years 2013, 2014, 2015, 2016 & 2017
| Year | Paper | Candidature | Maximum score | Mean Score | Standard Deviation |
| 2013 | 1 2 3 Overall |
439,787 439,770 439,765 439,847 |
80 80 40 200 |
16.68 18.31 14.67 49.00 |
13.89 14.25 5.68 32.10 |
| 2014 | 1 2 3 Overall |
476,582 |
80 80 40 200 |
25.44 21.33 17.57 64.31 |
15.79 13.46 6.19 35.63 |
| 2015 | 1 2 3 Overall |
515.840 515.803 515,799 515,888 |
80 80 40 200 |
26.83 22.07 20.37 68.71 |
15.78 13.45 7.15 33.29 |
| 2016 | 1 2 3 Overall |
566,836 |
80 80 40 200 |
19.15 14.66 13.63 47.42 |
14.85 12.85 6.31 |
| 2017 | 1 2 3 Overall |
606,515 |
80 80 40 200 |
17.03 17.97 14.1 48.09 |
14.67 14.32 6.11 32.87 |
From the table, it is to be seen that:
- Candidature for chemistry increased from 566,836 in 2016 to 606,515 in 2017 an increment of about 7%:
- Performance in paper 1 dropped with 2 units from a mean of 19.15 to a mean of 17.03. The drop was equivalent to 11.07%;
- Performance in paper 2 improved from a mean of 14.66 to 17.97. This was an improvement of 22.58%;
- Performance in paper 3 improved slightly from a mean of 13.63 to 14.1 an equivalence of 3.45%.
The slight improvement shows that students have taken charge of their learning and that the teachers have started teaching.
Questions which were performed poorly are briefly discussed below.

3.5.1 Chemistry Paper 1 (233/1)
The questions which were reported to have been poorly performed are briefly discussed below in view of pointing out the candidates' weaknesses and the proposed suggestions on the measures to be put in place in order to improve performance in future.
Question 3
The diagram in Figure 1 shows a section of a dry cell. Study it and answer the questions that follow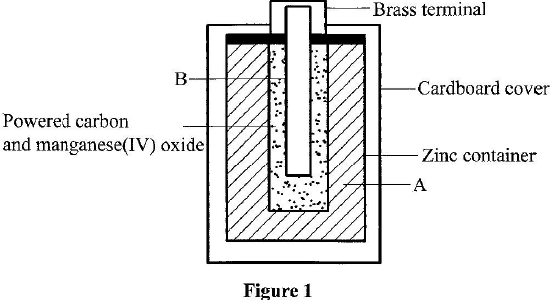
- Name the part labelled B. (1 mark)
- The part labelled A is a paste. Give a reason why it is not used in dry form. (1 mark)
- What is the purpose of the zinc container? (1 mark)
In this question, candidates were required to identify the parts of a dry cell and state their uses
Weaknesses
Most of the candidates could not identify and state the purpose of the parts of a dry cell.
Advice to teachers
Teachers should use practical approach when teaching the topic "Electrochemistry". Students should be allowed to carry out practical work on the dry cell or use demonstration.
Expected Response
- Carbon electrode (Anode)/Graphite electrode.
- To allow movement of ions/ to have it as an electrolyte. When dry, the ions are immobile.
- It is the cathode / negative electrode.
Question 8
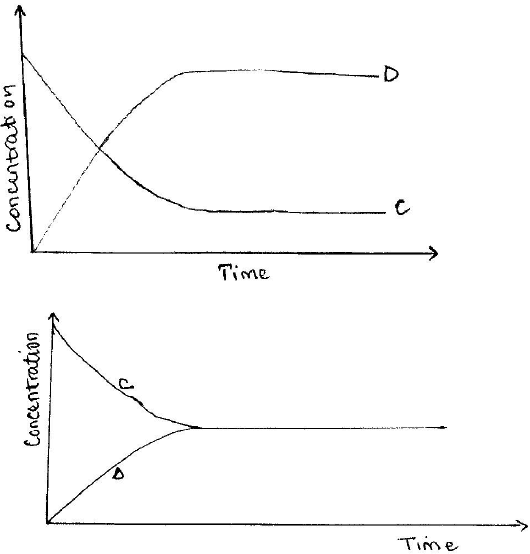
- State one characteristic of a reaction where equilibrium has been attained. (1 mark)
- The following equation is in a state of equilibrium: C <- ->D
Use it to sketch a graphical representation of concentration against time in seconds for the equilibrium. (2 marks)
In this question, candidates were required state the characteristic of a reaction at equilibrium and use a give equation to sketch a graphical representation of concentration against time.
Weaknesses
Most of the candidates did not know the characteristics hence could not know that at equilibrium, the curves flatten.
Expected Responses
- The concentrations of reactants and products remain constant or Rate of forward reaction is equal to the rate of backward reaction.
Question 12
Potassium nitrate liberates oxygen gas when heated. Draw a diagram of a set-up that shows heating of potassium nitrate and collection of oxygen gas. (3 marks)
The question was on preparation and collection of oxygen gas. The candidates were to draw a set up for preparation of oxygen from potassium nitrate.
Weaknesses
Most of the candidates could not draw labelled workable diagrams.
Expected response
- Heating - 1 mark
- method of collection - 1 mark
- workability-1 mark
Advice to teachers
Give students more practice on drawing of diagrams. Every experiment should be accompanied by a drawing illustrating the same.
Question 28
When an aqueous solution of compound X was mixed with a few drops of bromine water, the colour of the mixture remained yellow. When another portion of solution X was reacted with acidified potassium dichromate(VI), the colour of the mixture changed from orange to green.
- What conclusion can be made from the use of:
- bromine water? (1 mark)
- acidified potassium dichromate(VI)? (1 mark)
- Solution X was reacted with a piece of a metal and a colourless gas was produced. Describe a simple experiment to identify the gas. (1 mark)
This question was on test for unsaturation, reaction of ethanol with potassium manganate(VII) and reaction of ethanol with a metal.
Weaknesses
Most candidates were unable to make correct deductions using a given reagent and the observations.
Expected responses
-
-
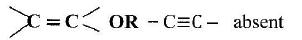
OR
Alkene, alkyne unsaturated hydrocarbon absent - -OH/R-OH present
-
- Lower a buming splint to the gas, a 'pop' sound should be produced showing it is hydrogen.
Advice to teachers
The topic should be taught using the practical approach and emphasis made on making correct inferences Teachers should empasise the use of correct technical terms when stating the inferences.
3.5.2 Chemistry Paper 2 (233/2)
Question 1
- Name the homologous series represented by each of the following general formulae.
- 0 CF 2 -2 - .................(1 mark)
- C12 ........... (1 mark)
- Compound G is a triester.
CH,(CH)COOCH
CH,(CH)COOCH
CH,(CH)COOCH
Compound G- Give the physical state of compound G at room temperature, (1 mark)
- G is completely hydrolysed by heating with aqueous sodium hydroxide.
- Give the structural formula of the alcohol formed. (1 mark)
- Write a fomula for the sodium salt formed. (1 mark)
- State the use of the sodium salt.
- Ethyne is the first member of the alkyne family. (1 mark)
- Name two reagents that can be used in the laboratory to prepare the gas (1 mark)
- Write an equation for the reaction. (1 mark)
- Perspex is an addition synthetic polymer formed from the monomer,
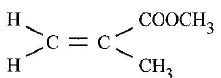
- What is meant by addition polymerisation?(1 mark)
- Draw three repeat units of perspex. (1 mark)
- Give one use of perspex. (1 mark)
- State two environmental hazards associated with synthetic polymers. (1 mark)
Weaknesses
Most candidates were unable to:
- give the structural formula of alkanol and formula of organic salt;
- state uses of organic compound;
- interpret the structure and draw structure of the polymer,
- write a balanced chemical equation;
- state uses of the polymer.
Expected responses
-
- CnH2n -alkyne
- CnH2n -alkene
-
- Solid because it is saturated.
-
- CH2OHCHOHCH2OH
- CH3(CH2)16COONa
- Cleaning agent / cleansing agent
-
- Calcium carbide and water.
- CaC2(s)+ H2O(l) + Ca(OH)2(aq) +C2H2(g)
-
- When many unsaturated molecules called monomers combine to form a
giant/macro molecule of high relative molecular mass called a polymer. -
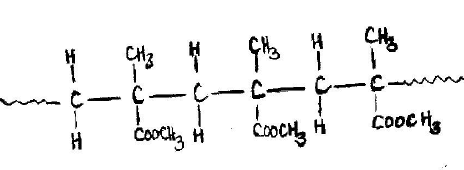
-
- Used as a glass substitute;
- Electronic instruments housing:
- Wind screen;
- Safety glasses:
- vehicle headlamps
- Bulletproofing
-
- Emit toxic fumes when burned affecting human life.
- They are non-biodegradable hence pollutes the environment
- Accelerates fires when burned/highly flammable.
- When many unsaturated molecules called monomers combine to form a
Advice to teachers
Teachers should approach this topic by use of real life examples of items made of polymers. Teachers should expose students to a lot of practice using questions. Students should be encouraged to do a lot of revision in organic chemistry.

3.5.3 Chemistry Paper 3 (233/3)
The practical paper was tested using three questions.
Question 1 tested knowledge, skills and competencies on:
- Measuring accurately volumes of solutions;
- Recording accurately such measured volumes
- Measuring and recording times using clocks;
- Measuring and recording temperatures:
- Interpreting results from a table;
- Titrations and recording of correct titre values;
- Simple Molar Calculations;
- Selection of suitable apparatus.
All the above mentioned skills and competencies were found to have been acquired by a large number of candidates. Calculations which have been a major challenge in the past were not a big challenge anymore. Mastery of Concepts is quite high.
However question 2 was identified to have been difficulty to a large number of candidates. This question was on qualitative analysis of an inorganic compound where the candidates were expected to design ar experiment from a given set of chemicals and materials
Question 2
You are provided with:
- Solid K
- Aqueous ammonia
- Aqueous sodium sulphate
- Dilute nitric(V) acid
- Wooden splint
Solid K is suspected to be lead(II) carbonate.
- From the reagents provided, select and describe three tests that could be carried out consecutively to confirm if solid K is lead(II) carbonate. Write the tests and expected observations in the places provided.
-
Test 1 Expected Observations -
Test 2 Expected Observations -
Test 3 Expected Observations
-
- Carry out the tests described in (a) using solid K and record the observations and Inferences in the spaces provided.
- Test 1
Observations Inferences - Test 2
Observations Inferences - Test 3
Observations Inferences
- Test 1
The question was based on project work and laboratory experimerts. This question required candidates to select and describe three tests that could be carried out consecutively to confirm the identity of solid.
This question is based on three general objectives of the KCSE Chemistry syllabus that by the end of the course:
- the learner should be able to select and handle appropriate apparatus for use in experimental work:
- make accurate measurements, observations and draw logical conclusions from experiments;
- observe and appreciate the need for safety precautions during experimental investigations
Weaknesses
Most candidates were unable to write the procedure in that they lacked the knowledge on solubility of salts. They were unable to make a solution containing Pb2+ ions from the given reagents hence could not carry out consecutive tests to identify the ions present in the solid.
| Test 1 | Expected Observations | |
| (i) | To solid K in a boiling tube, add about 10cm dilute nitric(V) acid. Retain mixture for test 2 & 3. Test any gas produced using a wooden splint. |
Expected Observations Effervescence / bubbles of gas or fizzing. Colourless gas extinguishes a burning splint. |
| Test 2 | Expected Observations | |
| (ii) | To about 2cm3 of mixture, add aqueous ammonia until in excess |
White precipitate insoluble in excess |
| Test 3 | Expected Observations | |
| (iii) | To about 2cm3 of mixture add 2 drops of aqueous sodium sulphate. |
White precipitate |
| (i) | Observations | Inferences |
| Test 1 | ||
| Effervescence, colourless gas extinguishes burning splint | CO32- present. Accept CO32-, written in words: |
|
| (ii) | Test 2 | |
| Observations | Inferences | |
| White precipitate insoluble in excess. | Mg2+, Pb2+ present | |
| (iii) | Test 3 | |
| Observations | Inferences | |
| No white precipitate. | Pb2+ absent OR Mg2+ present |
|
Advice to teachers
Teachers should make use of inquiry based learning approach in teaching qualitative analysis. Emphasis should be on the correct choice of apparatus and the use of correct scientific terms in reporting observations and inferences.
Question 3 tested on Skills and knowledge on qualitative analysis involving organic compound Candidates were able to record their observations well. However, some of them were not able to logically communicate their observations.
CONCLUSION
Chemistry as a subject was not performed well. Performance stood at a mean of 48.09. Of the three papers, practical which tests manipulative skills was the best with the mean standing at 35%. This means that a lot is required from both the learner and the teacher. Teachers should therefore do all they can to ensure that concepts, necessary skills and competencies are attained by the learners by the time they are sitting for their end of course examinations. This can easily be achieved by adopting the practical approach method in teaching the subject. Heads of Institutions, National County and Sub-Counties should ensure that the laboratories are well equipped for teaching/learning and for examination purposes
Download CHEMISTRY PAPER REPORT - KCSE 2017 REPORTS.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

