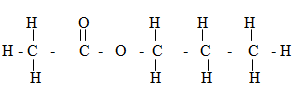INSTRUCTIONS
- Answer all the questions
- All working must be shown clearly where necessary
- Mathematical tables and silent non – programmable electronic calculators may be used
- Candidates must answer the questions in English.
- Candidates should check the question paper to ascertain that all the pages are printed as indicated and that no questions are missing.
- An element T has an atomic number 12.
- Write the formula of the nitride of T. (1mk)
- What type of bond is formed between T and Nitrogen (1mk)
- Ethanol has a lower molecular mass than butane.
Explain why ethanol is a liquid and butane is a gas at room temperature (2mks) - The set up below shows how a pure sample of Ethyne gas is prepared in the laboratory
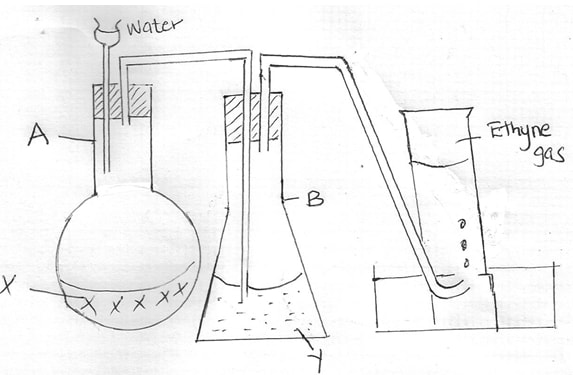
- Identify substances (1mk)
X ____________________________________
Y _____________________________________ - Write down a chemical equation for the reaction that takes place in flask A (1mk)
- Identify substances (1mk)
- A gas occupies 500cm2 at a temperature of 270C and 760mmHg pressure. Calculate its volume when pressure is doubled and temperature raised to 400C. (3mks)
- Chlorine gas is bubbled through Iron (II) sulphate solution.
- Explain the observations made (2mks)
- Write an ionic equation for the reaction that occurs (1mk)
- Study the scheme below and answer questions that follow
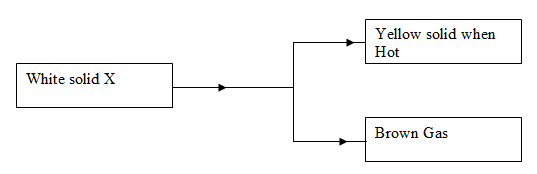
- Identify cations and anions in solid X (1mk)
Cations __________________________________
Anions ___________________________________ - Write down the equation for the reaction between hydrochloric acid and the yellow solid. (1mk)
- Identify cations and anions in solid X (1mk)
- Silicon and Sulphur are both non-metals in period 3. The melting points of their oxides are shown below
Explain this great difference in the melting point of the compounds. (2mks)Oxide Mpt oC SiO2 1728 SO2 -76 - Describe how you would obtain a pure sample of Sodium Carbonate from a mixture of Calcium Carbonate, Sodium carbonate and Ammonium Chloride. (3mks)
- Manganate (VII) ions react with Iron (II) ions in the presence of H+ ions according to the following reaction
MnO4- + 5Fe2+ + 8H+ → Mn2+ + 5Fe3+ + 4H2O- Work out the oxidation number of Manganese ions in the manganate (VII) ions (2mks)
- Which species has been reduced? Explain (2mks)
- Dry Chlorine gas was passed over hot iron wire as shown in the diagram below
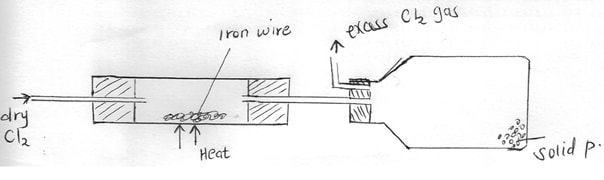
- Name solid P (1mk)
- Write a chemical equation for the reaction that forms solid P (1mk)
- The table below shows elements W,X,Y,Z in the same group of the periodic table. The letters do not represent the actual symbols of the elements. Study it to answer the questions that follow.
Element
Atomic radius (nm)
Ionic radius (nm)
W
0.072
0.136
X
0.133
0.216
Y
0.144
0.195
Z
0.099
0.181
- Is this a group of metallic or non - metallic elements? Explain (2mks)
- Arrange the elements in order of the reactivity starting with the least reactive (1mk)
- Use the following half cells standard reduction potentials to answer the questions that follow
Mg2+ + 2e- → Mg −2.36
Zn2+ + 2e- → Zn −0.76
Ag+ + e- → Ag +0.80
Cu22+ + 2e- → Cu +0.34- Select two half cells which when combined give the largest cell potential (1mk)
- Calculate the cell potential of the cell formed in a) above. (2mks)
- Concentrated Sulphuric (VI) acid is used as a drying agent. Explain why it is not suitable drying agent in preparation of Ammonia (2mks)
- Use the bond euthalples given below to answer the questions that follow
Calculate the heat of reaction ofBond
Bond euthalpies (KJ/Mol)
H-H
436
N-H
388
N=N
944
- N2(g) + 3H2(g) → 2NH3(g) (2mk)
- Is the above reaction exothermic or endothermic? Give a reason (1mk)
- The compound shown below is formed when an alcohol reacts with an alkanaic acid in presence of concentrated Sulphuric (VI) acid
- Give the IUPAC name of the compound (1mk)
- Identify the alcohol and the alkanoli acid that were used to form the compound
Alcohol __________________________
Alkanolic Acid _______________________
- What mass of copper would be deposited when a current of 2 amperes is passed for 4 minutes (1F = 96500 Coulombs, Cu= 63.5) (3mks)
- Starting with Copper metal, describe how we can prepare Copper (II) Carbonate. (3mks)
- When excess Magnesium ribbon is burned in air, two products are formed.
- Identify the two products (1mk)
- Write two equations for the reactions that form the two products in( i) above (2mks)
- Diamond and graphite are two allotropes of carbon
- Define the term allotrope (1mk)
- In terms of bonding, explain why graphite conduct electricity while diamond does not (2mks)
- 1.23g of the hydrated salt, MgSO4. XH2O was dissolved in 50cm3 of water and the solution diluted to 500cm3 using distilled water. The concentration of the final solution was found to be 0.011M.
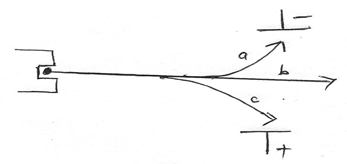
Find the value of X (Mg = 24, S=32, O=16, H=1) (3mks) - The arrangement below was used to compare the effects of an electric field on the emission of a radioactive decay.
- Name radiations (2mks)
a ____________________________________
b ____________________________________
c_____________________________________ - Account for the difference in the deflection of a and c (1mk)
- Name radiations (2mks)
-
- State the industrial method for the manufacture of ethanol (1mk)
- State two uses of ethanol (1mk)
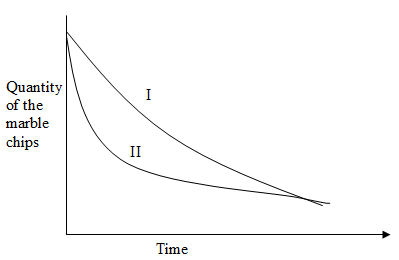
- The curves below were obtained when equal volumes of hydrochloric acid of the same concentration were reacted with 25g of marble chips. In one case, the acid was first warmed to a higher temperature.
- Which curve represents the reaction using warm hydrochloric acid? (1mk)
- Explain how temperature affects the rate of reaction. (1mk)
- Apart from temperature, state any other factor that affects the rate of reaction involving solids. (1mk)
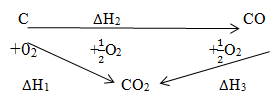
- Study the energy cycle diagram below and then answer the questions that follow.
- Name the heat changes labeled ∆H2 and ∆H3. (1mk)
- Given that the ∆H2 = -110kJMol-1 and ∆H3 = -283kJMol-1 . Calculate the value of ∆H1 for one mole of graphite C = 12, O=16 (2mks)
- Ammonium Iron (II) Sulphate heptahydrate was heated gently then strongly in a dry test tube. Explain the observations made. (3mks)
- A Solution contains 4g of Potassium hydroxide in one litre. 25.0cm3 of this solution requires 22.0cm3 of dilute SulphuricVI acid for complete neutralization. Calculate the concentrationof the acid in moles per litre. (K=39, O = 16) (3mks)
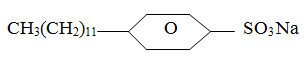
- The following structure represents a popular compound used for washing
- Explain why it is a better washing agent than soap in both hard and soft water (2mks)
- State one problem associated with the use of the above compound. (1mk)
-
- State two properties of carbon(IV) oxide that makes it suitable for use as a fire extinguisher (1mk)
- Give one use of Carbon (IV) oxide other than as fire extinguisher (1mk)
- Ethanoic acid has a higher boiling point than ethanol. Explain this in terms of structure and bonding. (2mks)
- Drug abuse is a rapidly growing problem.
- Name two addictive drugs (1mk)
- State one problem that drug above can cause. (1mk)
Download CHEMISTRY PAPER 1 - 2019 KCSE Prediction Questions Set 2.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students