-
- Study the information in the table below and answer the questions that follow.
(The letters do not represent the actual symbol of the substances).
Substance Density at room temperature g/cm3 Solubility in water Boiling point (oC) Melting point(oC) U 0.8 Very soluble 78.5 -117 V 0.77 × 10-3 Very soluble -33 -78 W 1.6 Insoluble 77 -23 X 1.33 × 10-3 Slightly soluble -183 -219 - Select the letter that represents a substance that is a gas at room temperature and which can be collected:
- Over water. Explain. {2 marks}
- By downward displacement of air. (Density of air is 1.29 x 10-3 g/cm3 at room temperature). {1 mark}
- Which substance would dissolve in water and could be separated from the solution by fractional distillation? Explain. {2 marks}
- Which substance is a liquid at room temperature and when mixed with water two layers would be formed? {1 mark}
- Select the letter that represents a substance that is a gas at room temperature and which can be collected:
- The grid below shows part of a periodic table. Study it and answer the questions that follow.
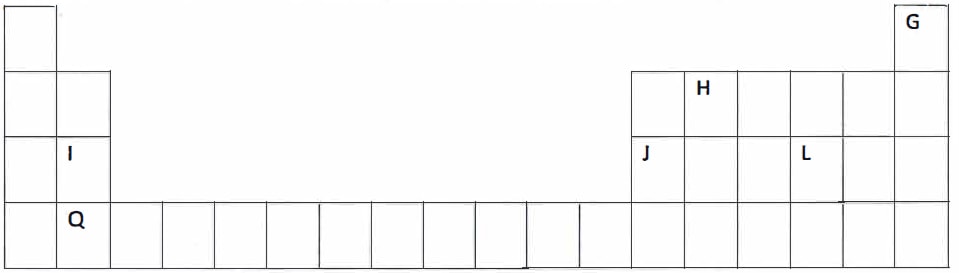
(The letters do not represent the actual symbols of the elements)- Name the Group to which element I and Q belong. {1 mark}
- Which letter represents the element that is least reactive? {1 mark}
- Name the type of bond that is formed between element H and L when they react. Explain. {2 marks}
- Write the chemical formula of the compound formed when element J and Oxygen gas react. {1 mark}
- On the grid, indicate with a tick (√) the position of element R, which forms R3- ions and is in the third period of the Periodic Table. {1 mark}
- Study the information in the table below and answer the questions that follow.
-
- A form four student investigated a property of acids C and E by reacting equal volumes of 1 M sodium hydroxide solution with equal volumes of acid C and E of the same concentration. The results obtained were recorded in the table below.
Acid C E Rise in temperature (Δt)k 4 2 - Select the letter that represent a weak acid. Explain. {2 marks}
- Write a chemical equation for the reaction between Sodium hydroxide solution and aqueous ethanoic acid. {1 mark}
-
- What is molar heat of vaporization? {1 mark}
- The formation of Hydrogen peroxide under standard conditions is represented by the following thermochemical equations:
H2(g) + O2(g) → H202(g) ΔHθf = -133 kJ/mol
H2(g) + O2(g) → H202(l) ΔHθf = -188 kJ/mol
Draw an energy cycle diagram that links the above two thermochemical equations. {3 marks} - Calculate the molar heat of vaporization of hydrogen peroxide. {1 mark}
- Write the thermochemical equation for the molar heat of vaporization of Hydrogen peroxide. {1 mark}
-
- What is a fuel? {1 mark}
- Natural gas, coal and oil are known as fossil fuels. They are major sources of energy.
Hydrogen gas is also a source of energy.- State and explain two reasons why Hydrogen is a very attractive fuel compared to fossils. {2 marks}
- State one disadvantage of using Hydrogen fuel instead of fossil fuels.{1 mark}
- A form four student investigated a property of acids C and E by reacting equal volumes of 1 M sodium hydroxide solution with equal volumes of acid C and E of the same concentration. The results obtained were recorded in the table below.
- The flow chart below is a simplified version of the steps in the manufacture of Sodium carbonate.
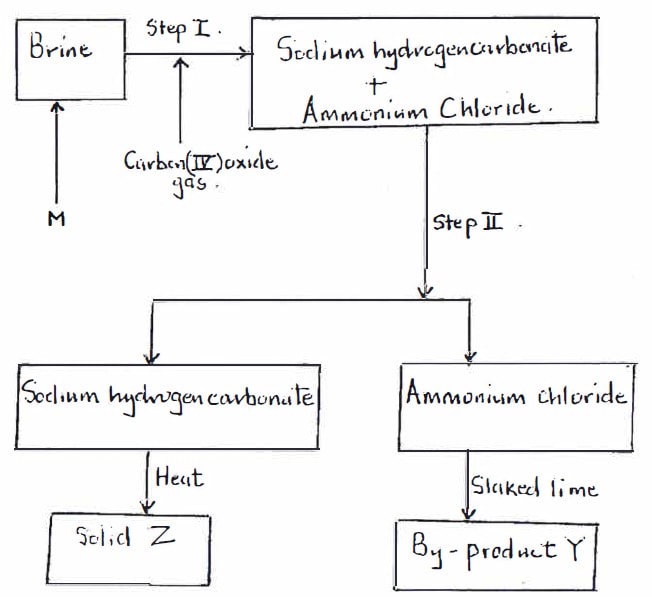
- Name the process illustrated by the flow chart above. {1 mark}
- Name three main raw materials of the above process. {3 marks}
- Name substance M. {1 mark}
- Name the process taking place in step II. {1 mark}
- Identify by-product Y and write a balanced chemical equation to show how it is formed. {2 marks}
- 98.123 Kg of Sodium hydrogen carbonate was manufactured in this process.
- What is solid Z. {1 mark}
- Write a chemical equation for the formation of solid Z. {1 mark}
- Determine in Kilograms, how much of solid Z was produced in this process. (Na = 23, H = 1, 0 = 16, C = 12) {3 marks}
-
- Study the standard reduction potentials for elements R, T, U, V and W given below and answer the questions that follow. (The letters are not the actual symbols of the elements).
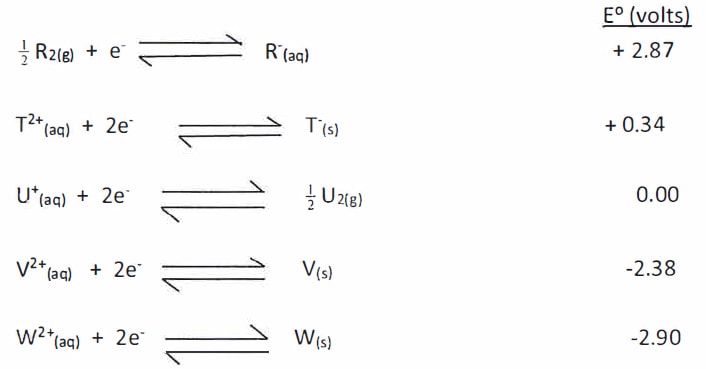
- What is the E0 value of the strongest reducing agent? {1 mark}
- Which element is likely to be Hydrogen? Give a reason for your answer. {2 marks}
- Draw in the space provided below, a labelled diagram of the electrochemical cell that would be obtained when half-cells of elements T and V are combined. {3 marks}
- On the diagram (iii) above:
- draw an arrow showing direction of flow of electrons. {1 mark}
- Label by naming the anode and cathode of the electrochemical cell.{1 mark}
- Determine the E0 value of the electrochemical cell constructed in (iii) above.{2 marks}
- 1.48 g of Copper metal were deposited when current was passed through aqueous Copper (II) Sulphate for 2 hours 30 minutes during purification of Copper in electrolysis. Determine the amount of current used. (Cu= 63.5, IF= 96500 C) {3 marks}
- Study the standard reduction potentials for elements R, T, U, V and W given below and answer the questions that follow. (The letters are not the actual symbols of the elements).
-
- The following scheme represents various reactions starting with pro-1-ene. Use it to answer the questions that follow.
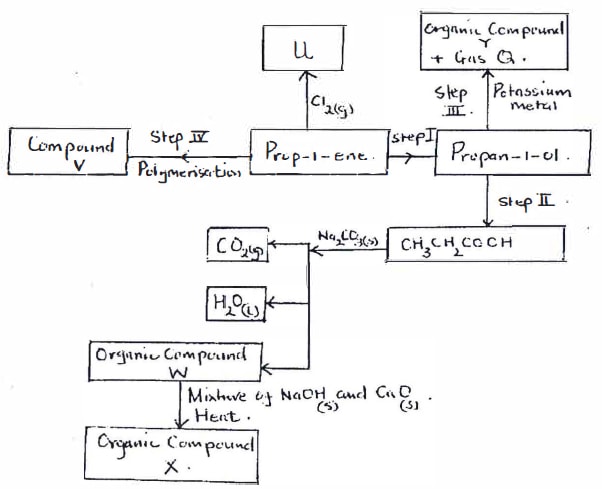
- Write the chemical name of Organic compound X. {1 mark}
- Write the formula of organic compound Y. {1 mark}
- State one reagent that can be used in:
- Step I {1 mark}
- Step II {1 mark}
- Name compound V and state one of its use. {1 mark}
-
- Name gas Q. {1 mark}
- Write the equation of the reaction in step III {1 mark}
- Name organic compound Y. {1 mark}
- The structure shown below represents the monomer of natural rubber.
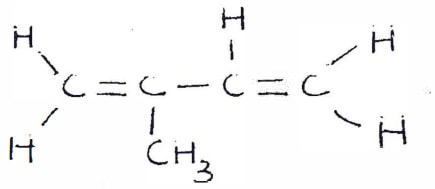
Using two monomers of the above structure, show how the monomer polymerises. {1 mark}
- The following scheme represents various reactions starting with pro-1-ene. Use it to answer the questions that follow.
- Below is a simplified diagram of a blast furnace used in the extraction of iron from its ores. Study it and answer the questions that follow.
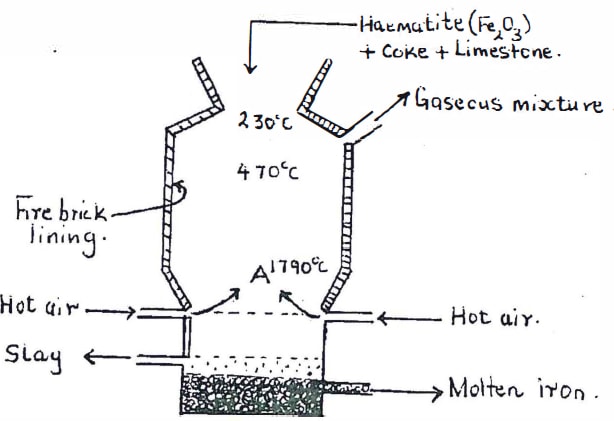
- Name one other iron ore material that can be used in the blast furnace. {1 mark}
- The iron obtained from the blast furnace has a melting point of 1200°C while that of pure iron is 1535°C. Explain. {1 mark}
- What is the purpose of limestone in the blast furnace? {1 mark}
- State one physical property of molten slag other than density that allows it to be separated from molten iron as shown in the diagram above. {1 mark}
- State one use of fire-brick lining in the blast furnace. {1 mark}
-
- Name one of the reducing agents used to reduce iron {III) oxide to iron metal in the blast furnace. {1 mark}
- Using the named reducing agent in f(i) above and iron (III) oxide, write an equation involving this reaction. {1 mark}
- Explain why the temperature in the region marked A is higher than that of the incoming hot air. {1 mark}
-
- What name is given to the 90- 95% pure iron obtained from the blast furnace? {1 mark
- lron from the blast furnace contains about 5% carbon. State how the carbon content is reduced. {1 mark}
-
- A sample of water containing 0.001 moles of calcium hydrogencarbonate was added to 90 cm3 of 0.01M calcium hydroxide.
- Write an equation for the reaction that took place. {1 mark}
- Calculate the number of moles of calcium ions in 90 cm3 of 0.01M calcium hydroxide. {1 mark}
- What would be observed if soap solution was added dropwise to a sample of the water that had been added to calcium hydroxide. Give a reason. {2 marks}
- The column below was used to soften hard water.
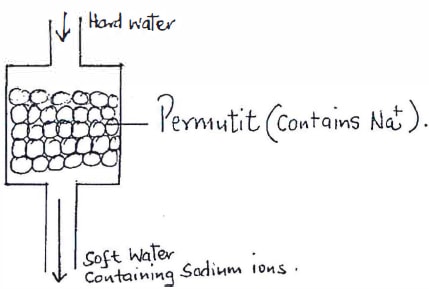
- Explain how the hard water was softened as it passed through the column.{1 mark}
- After sometime the material in the column is not able to soften hard water. How can the material be regenerated?
- State one disadvantage of using hard water in boilers. {1 mark}
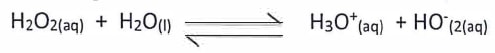
- Identify the reagent that acts as a base in the equation below. Give a reason. {2 marks} {1 mark}
- A sample of water containing 0.001 moles of calcium hydrogencarbonate was added to 90 cm3 of 0.01M calcium hydroxide.
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download CHEMISTRY PAPER 2 - 2019 K.C.SE Prediction Questions Set 1.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

