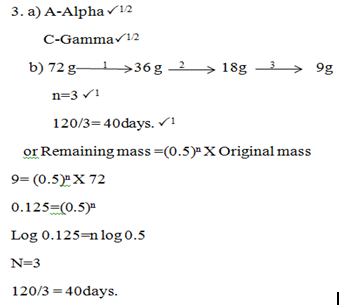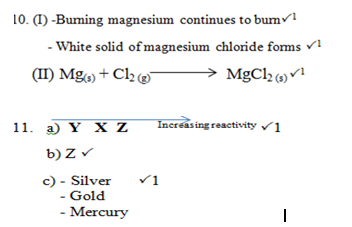- Explain why aluminium is a better conductor of electricity than magnesium. (1 mark)
- Other than cost and ability to conduct, give two other reasons why aluminum is used for making overheadelectriccables while magnesium is not. (1mark)
- In the Haber process, the industrial manufacture of ammonia is given by the following equation:
- Name one source of hydrogen gas used in this process. (1 mark)
- Name the catalyst used in the above reaction. (1mark)
- State any two uses of ammonia. (1 mark)
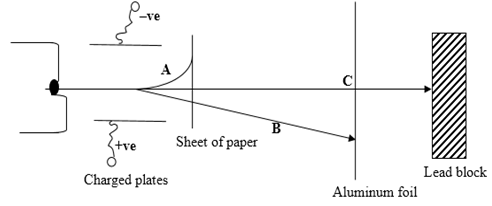
- Use the diagram below to answer the questions that follow:
- Identify the radiations. (1 mark)
- The table below gives the rate of decay for a sample of radioactive element K. Study it and answer the question that follows:
Determine the half-life of element K. (2 marks)Mass (kg)
Time(Days)
72
0
9
120
- The pH values of some solutions labeled K,H,L,P and R are given in the table below. Use the information to answer the questions that follow.
Identify the solution with the highest concentration of hydroxyl ions. (1 mark)pH
8.0
14.0
1.0
6.5
7.0
Solution
K
H
L
P
R
- Which solution is likely to be sodium chloride solution? (1 mark)
- Which solution would react most vigorously with magnesium metal? (1 mark)
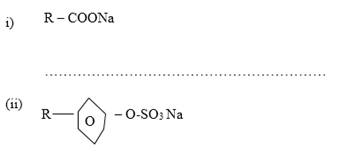
- Name the class to which the following cleansing agents belong: ( 1mark)
- Which cleaning agent between (i) or (ii) above is preferred for cleaning garments while using water from a dam containing dissolved calcium chloride? Explain (2 marks)
- Name the class to which the following cleansing agents belong: ( 1mark)
- Describe how crystals of sodium chloride can be prepared starting with 50cm3 of 2M sodium hydroxide solution. (3marks)
- Propane (C3H8) and Carbon(IV)oxide (CO2) diffuses at the same rate under the same conditions. Explain. (1 mark)
- Propane is a hydrocarbon. What does the term hydrocarbon mean? (1 mark)
- The reaction between hydrochloric acid and potassium dichromate (VI) can be used to demonstrate a reversible reaction. The ionic equation is given below
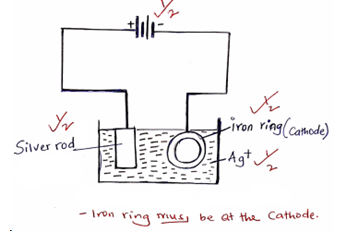
Explain the observation that would be made when few drops of dilute hydrochloride acid is added to the equilibrium mixture. (2marks) - Draw a diagram to show how an iron ring can be electroplated with pure silver. (2marks)
- Give two reasons why electroplating is necessary. (1 mark)
- State two observations that can be made when burning magnesium is lowered in a gas jar full of chlorine. (2 marks)
- Write a chemical equation for the reaction that occurs in (i) above. (1 mark)
- The products formed by action of heat on nitrates of element X, Y and Z are shown below.
Nitrate of
Products formed
X
X oxide +Nitrogen (IV) Oxide + Oxygen
Y
Y +Nitrogen (IV) Oxide+ Oxygen
Z
Z nitrite + oxygen
- Arrange the metals in order of increasing reactivity. (1 mark)
- Which element forms a soluble carbonate? (1mark)
- Give an element that can beY. (1 mark)
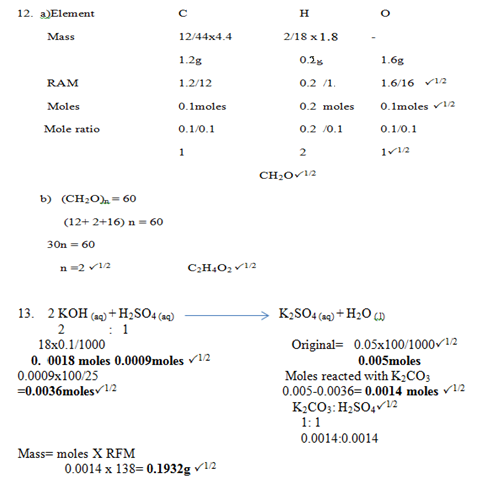
- 12.3.0g of an organic compound containing Carbon, hydrogen and oxygen only produced 4.4g of Carbon (IV) oxide and 1.8g of water on complete combustion.
- Calculate its empirical formula. (2 marks)
- Calculate in molecular formula if its formula mass is 60. (1 mark)
- 100cm3 of0.05M Sulphuric (VI) acid were placed in the flask and small quantity of potassium Carbonate added. The mixture was boiled to expel all carbon (IV) oxide. 25cm3 of the resulting solution required 18cm3 of 0.1M potassium hydroxide solution to neutralize it. Calculate the mass of potassium carbonate added. (K = 39, O = 16, C = 12) (3 marks)
- A sample of air contains nitrogen, oxygen and argon. Describe how oxygen gas can be obtained. (3 marks)
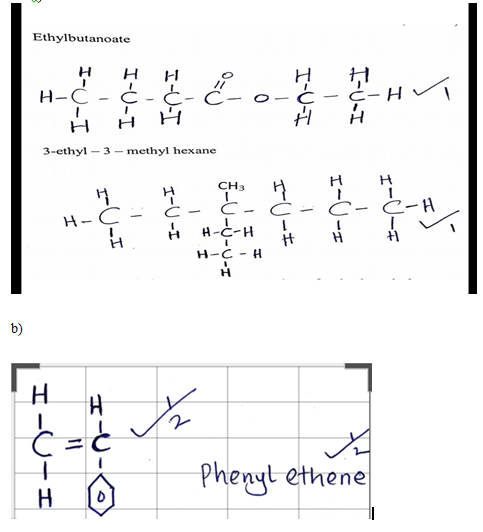
- Draw the structures of the following compounds. (2 Marks)
- Ethylbutanoate
- 3-ethyl – 3 – methyl hexane
- Draw the structures of the following compounds. (2 Marks)
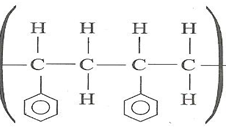
- The structure below represents a portion of polyphenylethene.
Draw the structure of the monomer and name it. (1mark) - A compound whose general formula is P (OH) 3 (s)reacts as shown by the equation:
P (OH) 3(s) + OH-(aq) P (OH) 4-(aq)
P (OH) 3(S) + 3H+ (aq) P3+ (aq) + 3H2O (l)- Name any two elements whose hydroxides behave like P. (2 marks)
- What name is given to compounds which behaves like P (OH) 3 in the above two reactions. (1 mark)
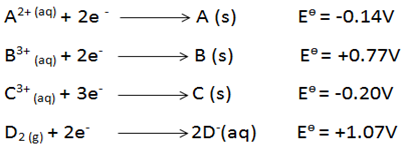
- You are provided with the following electrode potentials of four half-cell reactions. Letters do not represent the actual symbol of the element.
- Identify the strongest reducing agent. Explain. (2marks)
- Calculate the emf of the two half cells that when combined would produce the largest emf. (1 mark)
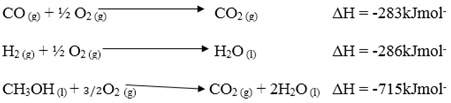
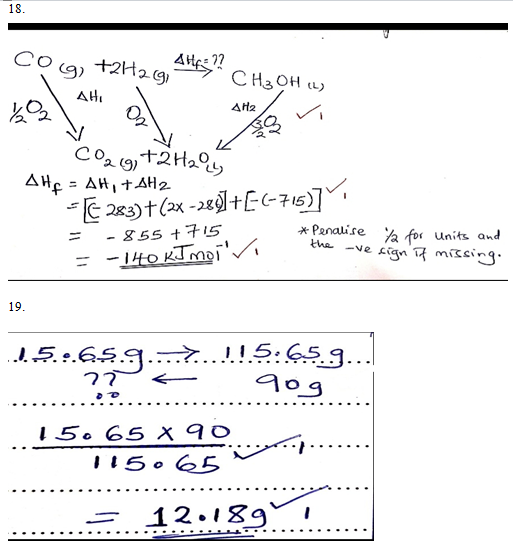
- Use the following information to answer the questions that follow.
- Draw an energy cycle diagram and label the various heat changes. (2 marks)
- Use the energy circle above to calculate heat of formation of methanol. (1 mark)
- The solubility of iron (II) sulphate at 22oC is 15.65g/100g of water. Calculate the mass of iron (II) sulphate crystals in 90g of saturated iron (II) sulphate solution. (2marks)
- Which Use the information in the table below to answer the questions that follow.
(The letters do not represent the actual symbols of the elements).
Give the number of neutrons in an atom of element R 1mkElement
Q
P
R
S
T
Atomic number
18
5
3
5
20
Mass number
40
10
7
11
40
- Which two letters represent the same element. 1mk
- A solid sample was suspected to contain zinc (II) ions. Describe a systematic test to confirm the presence of zinc (II) ions. (3 Marks)
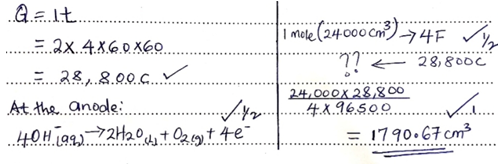
- During the electrolysis, a current of 2 amperes was passed through the copper (II) sulphatesolution for 4 hours. Calculate the volume of the gas produced at the anode. (1 F= 96500 C, MGV = 24000cm3). (3 marks)
- A volume of 10cm3 of ethene gas (C2H4) was exploded with 50cm3 of oxygen.
- Write the equation of the reaction for the combustion of ethene. (1mk)
- Calculate the volume of gaseous mixture. (2marks)
- What is a fuel? (1 mark)
- Ethanol has a molar heatof combustion of -1360kJmol-1. Calculate the heating value of ethanol. (C=12,H=1,O=16) (2 marks)
- Why is the percentage of carbon (IV) oxide in the atmosphere fairly constant? (1 mark)
- Calculate the volume of carbon(IV)oxide in 8,000 m3 of air contained in a hall.(2 marks)
- State two conditions that would make the boiling point of water to be higher than 100oC. (2marks)
- Name two substances that can be used in chemical test for water. (1mark)
- Describe how a student can determine the purity of tap water in a school laboratory (2marks)
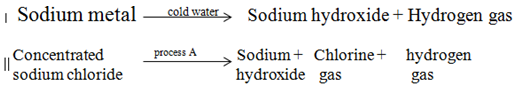
- Sodium hydroxide can be prepared through the following methods: I and II.
- Name one precaution that needs to be taken in method I. ( 1 mark)
- Give the name of process A. (1 mark)
- Describe the chemical test of hydrogen gas. (1 mark)
- What does CFCs mean? (1 mark)
- State one environment effect of CFCs. (1 mark)

MARKING SCHEME
- Aluminium has three delocalized electrons that conducts electricity while magnesium has only two. P1
- Aluminium forms a layer of oxide that is unreactive hence prevents it from corrosionP1/2
Aluminium is lighter than magnesiumP1/2
- -Cracking of long chain alkanes
- Electrolysis of brineP1 Any one correct.
-From natural gas - Finely divided iron catalyst P1
- -Used as a fertilizer
- Manufacture of nitric (V) acid any two correctP1/2 each.
- Manufacture of nitrogenous fertilizer
- Removing greasy stains
- As a refrigerant in warehouses
- -Cracking of long chain alkanes
- H/14.0P1
- R /7.0P1
- L/1.0P1
- Soapy detergent P1/2
- Soapless detergent P1/2
- Detergent(II) /soaplessP1
Doesn’t not form scum with hard water. P1
- -Add 50cm3 of 2M sodium hydroxide solution to a beaker containing 50cm3 of 2M of dilute hydrochloric acid. P1 * If right quantities not indicated, penalize 1 mark
-Stir the solution well then heat to saturationP1
-Allow the saturated solution to cool slowly to form crystalsP1/2
-Dry the crystals of sodium chloride P1/2 - This is because they have same molecular mass hence same rate of diffusion. P1
- Hydrocarbon- compounds that contains hydrogen and carbon atoms only. P1
- Orange colour intensifies P1
Adding drops of hydrochloric acid increases the concentration of H+ hence more CrO42- reacts favoring forward reaction. P1
To improve appearance P1/2
To prevent corrosion P1/2
- .-Compress the gaseous mixture to a pressure of 200 atmosphere and cool it to -200oC.P1
- Carry out fractional distillation.P1
- Collect the gas obtained at -183oCP1
- - Zinc P1
- Lead P1
- Aluminum - Amphoteric compounds P1
- - Zinc P1
- C (s)P1
Has the highest tendency to lose electronsP1 - Emf= Ered -Eox
(+1.07-(-0.20) P½
= +1.27V P½ 1Penalise ½ if +ve sign missing.
- C (s)P1
- P and SP1
- 7-3=4. P1
- – Add water to dissolveP1
- Add ammonia solution dropwise till excess.P1
- White precipitate that dissolves in excessP1
- C2H4(g) + 3O2(g)2CO2(g) +2 H2O(g) P1
- C2H4(g) + 3O2 (g)2CO2 (g) +2 H2O (g)
13210cm330cm3 20cm3*Using moles ratios for volume ratio
Volume of oxygen that remained: 50-30=20cm3P1
Gaseous mixture: 20+20 = 40cm3P1
- Fuel: A substance that produce useful heat energy when it undergoes a chemical or a nuclear reactionP1
- Heating Value= Molar Heat of Combustion/ RFM
1360/46P1
= 29.57 kJg-P1
- The natural processes that emit carbon (IV) oxide and those that absorbs it almost progress at the same rate. P1
.
- – Increased pressure above 1 atmosphere.P1
- Presence of impurities.P1
- -Anhydrous copper (II) sulphate P1/2 *penalize fully if the word anhydrous missing
- Anhydrous cobalt (II) chlorideP1/2 - –Heat the waterP1/2 anddetermine the boiling point using a thermometerP1/2
- The water boils at a rangeP1/2 of temperatures above a 100OCP1/2
- -Anhydrous copper (II) sulphate P1/2 *penalize fully if the word anhydrous missing
- A small piece of sodium metal should be used because the reaction is violent/ use of eye protectionP1
- Electrolysis P1
- Introduce a burning splintP1/2 where the gas extinguishes the burning splint with a “pop”PP1/2
- ChlorofluorocarbonsP1
- It pollutes the environment by eroding the ozone layer.P1
Download CHEMISTRY PAPER 1 - 2019 LAINAKU JOINT MOCK EVALUATION EXAMINATION.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students