- Elements Q, S, T, U, R and P belong to the same period in the periodic table. The ions formed by the atoms of the elements are given below:
Q2+, U-,T 2+,R3+,P+and S3-- Arrange the elements in order of increasing atomic size. [2mks]
- Suggest a reason why elements P and Q cannot react with each other to form a compound.[1mk]
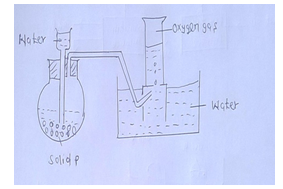
- The diagram below represents a set-up that can be used to prepare and collect oxygen gas.
- Name solid P. [1mk]
- What property of oxygen makes it possible for its collection as indicated by the diagram.[1mk]
- Explain why it is important not to collect any gas for the first few seconds of the experiment.[1mk]
- M grammes of a radioactive isotope decayed to 5 grammes in 100 days. The half-life of the isotopes is 25 days.
- What is meant by the term half-life? [1mk]
- Calculate the initial mass of M of the radioactive isotope. [2mks]
- Dilute hydrochloric acid is warmed with sodium sulphide
- Write an equation for the reaction involved. [1mk]
- State a chemical test for the gas evolved. [1mk]
- The system below is at equilibrium.
N2[g]+O2[g] ⇋ 2NO[g] H=+94KJ
Explain how an increase in the following affects the equilibrium position. [3mks]- Temperature
- Pressure
- When a current of 2.5 amperes was passed through a cell containing T2+ ions of a metal for 25 minutes, the mass of the cathode increased by 0.36g.Determine the relative atomic mass of element T.(IF=96500C). [3mks]
- Describe how you would prepare crystals of sodium nitrate starting with 200cm3 of 2m sodium hydroxide. [3mks]
-
- Name the polymer with the following structural formula.
-CH-CH2-CH-CH2-CH-CH2CH-CH2-
Cl Cl Cl Cl - State one commercial use of the polymer. [1mk]
- Name the polymer with the following structural formula.
- Drawdot (.)and cross(x) diagram to show bonding in carbon (II) oxide. [2mks]
- In one of the dry practical assignment to analyse cat ion in a salt, the following observations were made.
TEST
OBSERVATIONS
INFERENCES
[i]NaoH drop wise till in excess
White precipitate formed soluble in excess
[ii]NH3 solution drop wise till in excess
Presence of Zn 2+confirmed.
- Fill in the blanks in the table above. [2mks]
- Give an ionic equation for the reaction that occurs in test (ii) when excess ammonia solution is added. [1mk]
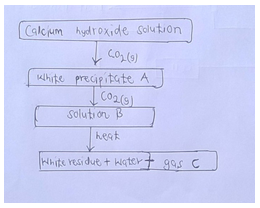
- Study the flowchart below and answer the questions that follow
Identify; [3mks]- White precipitate A
- Solution B
- Gas C
- Given the following electrode potentials;
A + + e- → A[s] +0.76V
B2+[aq]+2e- → B[s] -0.48V
Q2[g] +2 e- → Q2-[aq] +1.62V- Determine the maximum emf that can be obtained by combining two of the given half cells.[1mk]
- Write the cell representation for the cell in (a) above. [1mk]
- Use the bond energies given below to answer the questions that follow.
Bond Bond energy (KJMO1-1)
H-H 432
C=C 610
C-C 346
C-H 413
Determine the enthalpy change for the conversion of but-i-ene to butane by hydrogen. [3mks]
- The following data gives the pH values of some solutions A,Band C.
Solution Ph
A 13.0
B 6.9
C 2.0- Which solution would produce carbon (iv) oxide gas when reacted with copper (ii) carbonate.[1mk]
- What colour change would occur in solution A on addition of drops of phenolphthalein indicator.[1mk]
- What volume of 0.2 m hydrochloric acid would react completely with 0.005 moles pure calcium carbonate? [2mks]
- When an electric current was passed through molten substances P and Q in different containers, the observations below were made;
Molten P-Conduct electricity and is not decomposed.
Molten Q-Conducts electric current and a gas is formed at one of the electrodes
Suggest the type of bonding present in;- Substance P [1mk]
- Substance Q [1mk]
-
- Stat Gay-Cossack’s law. [1mk]
- 40cm3 of carbon (II) oxide and 40cm3 of oxygen were sparked in a closed vessel.
- Write a chemical equation for the reaction that occurs. [1mk]
- Determine the composition of the residual gases. [2mks]
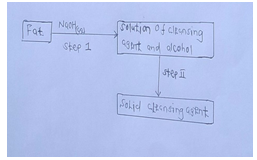
- The scheme below was used to prepare a cleansing agent. Study it and answer the questions that follow.
- Give the type of cleansing agent prepared by the above method. [1mk]
- Name the chemical substance added in step ii. [1mk]
- What is the purpose of adding the chemical substance named in [ii] above? [1mk]
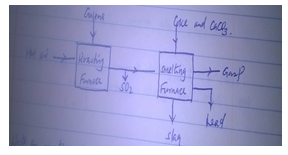
- During the extraction of lead from its ore, one of the main ore used is Galena as shown.
- Write an equation for the reaction in roasting furnace. [1mk]
- Name gas P. [1mk]
- State any two uses of lead metal. [1mk]
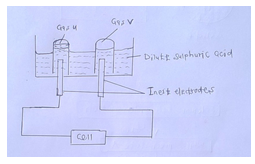
- The figure below shows the electrolysis of dilute sulphuric (VI) acid.
- On the diagram label the cathode and the anode. [1mk]
- Name the gases U and V. [1mk]
- Write the half cell equation for the reaction taking place at the anode. [1mk]
- The chemical equations below are the main reactions in large scale manufacture of sodium carbonate.
NH3 (g) +CO2[g] +H2O [l] → NH4HCO3[aq]
NH4HCO3[aq]+Nacl[aq] → NaHCO3+NH4Cl [aq]- Explain how the two products,NaHCO3and NH4Cl are separated. [1mk]
- How sodium carbonate is finally obtained? [1mk]
- State two uses of sodium carbonate. [1mk]
- 20cm3 of an unknown gas Q takes 12.6 seconds to pass through small orifice-10cm3 of oxygen gas takes 11.2 seconds to diffuse through the same orifice under the same conditions of temperature and pressure. Calculate the molecular mass of unknown gas Q. [3mks]
-
- Define the term isomerism. [1mk]
- Draw and name two positional isomers of butene. [2mks]
- Filter papers dipped in acidified potassium manganate (vii] were placed in two separate gas jars A and B containing pentane and pent-i-ene respectively. Explain what was observed in each case.[2mks]
- The following pairs of compounds were reacted together and the maximum temperature rise recorded for each reaction.
A-50cm3of 2m ammonia solution and 50cm3 of 2m ethanoic acid.
B-50cm3 of 2m sodium hydroxide solution and 50cm3 of 2m hydrochloric acid
C-50cm3 of 2m sodium hydroxide and 50cm3 of 2m elthanoic acid- State the pair which showed: [3mks]
- The highest temperature rise
- The lowest temperature rise
- Explain your answer above
- State the pair which showed: [3mks]
- Piecesof blue and red litmus papers were placed in a beaker containing water into which aluminium chloride had been dissolved.
- Is dissolving of aluminium chloride in water a physical or a chemical process? Explain. [2mks]
- State the observations made on the papers. Explain your answer. [2mks]
- A mass of 2.5g of acid Hx was dissolved in water and the resulting solution was diluted to a total of 250cm3.15cm3 of the final solution was diluted to a total of 250cm3.15cm3 of the final solution was required to neutralise 25.0cm3 of 0.1m aqueous potassium hydroxide. Calculate the relative molecular mass of the acid. [3mks]
- 75g of a saturated solution contains 3g of a certain salt. Calculate:
- The solubility of the salt. [2mks]
- The percentage of the salt in the saturated solution. [1mk]
- Using an energy cycle diagram, calculate the enthalpy change of formation of carbon disulphide.[3mks]
S[s]+o2[g] → so2[g] H=-294 KJmol-1
Cs2[s]+3o2[g] → co2[g]+2so2[g] H=-1072KJMOL-1
C[s]+o2[g] → co2[g] H=-393KJmol-1
Download CHEMISTRY PAPER 1 - KCSE 2019 MARANDA MOCK EXAMINATION.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students