- You are provided with;
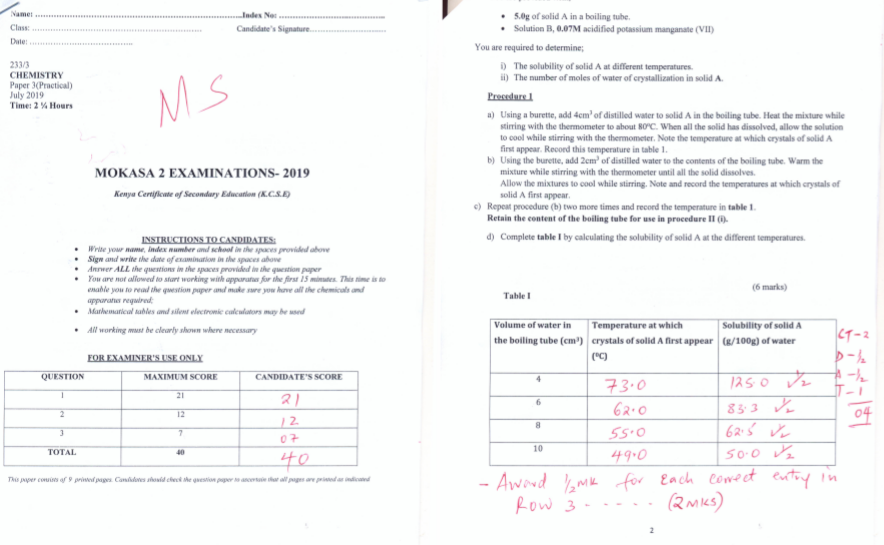
0g of solid A in a boiling tube.
Solution B, 07M acidified potassium manganate (VII)
You are required to determine;- The solubility of solid A at different temperatures.
- The number of moles of water of crystallization in solid A.
Procedure 1
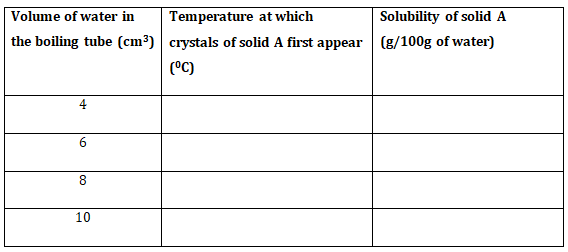
- Using a burette, add 4cm3 of distilled water to solid A in the boiling tube. Heat the mixture while stirring with the thermometer to about 80o When all the solid has dissolved, allow the solution to cool while stirring with the thermometer. Note the temperature at which crystals of solid A first appear. Record this temperature in table 1.
- Using the burette, add 2cm3 of distilled water to the contents of the boiling tube. Warm the mixture while stirring with the thermometer until all the solid dissolves.Allow the mixture to cool while stirring. Note and record the temperature at which crystals of solid A first appear.
- Repeat procedure (b) two more times and record the temperature in table 1.
Retain the content of the boiling tube for use in procedure II (i). - Complete table I by calculating the solubility of solid A at the different temperatures. (6 marks)
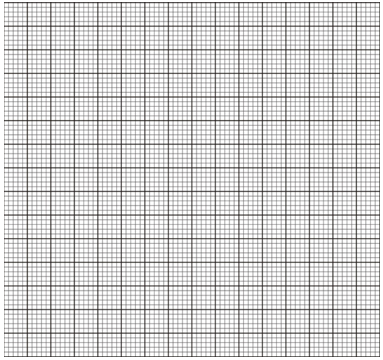
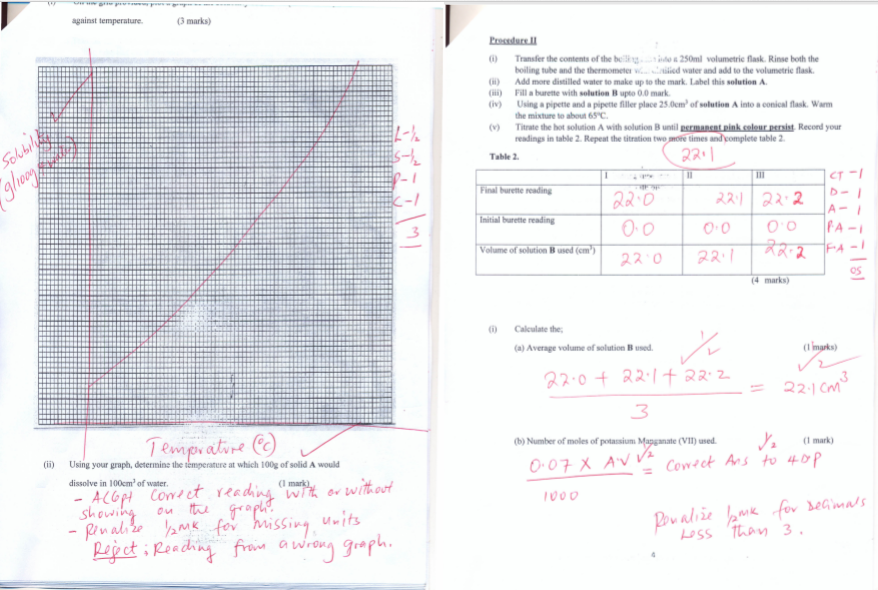
- On the grid provided, plot a graph of the solubility of solid A (vertical axis) against temperature. (3 marks)
- Using your graph, determine the temperature at which 100g of solid A would dissolve in 100cm3 of water. (1 mark)
- On the grid provided, plot a graph of the solubility of solid A (vertical axis) against temperature. (3 marks)
Procedure II
- Transfer the contents of the boiling tube into a 250ml volumetric flask. Rinse both the boiling tube and the thermometer with distilled water and add to the volumetric flask.
- Add more distilled water to make up to the mark. Label this solution A.
- Fill a burette with solution B upto 0.0 mark.
- Using a pipette and a pipette filler place 25.0cm3 of solution A into a conical flask. Warm the mixture to about 65o
- Titrate the hot solution A with solution B until permanent pink colourpersist. Record your readings in table 2. Repeat the titration two more times and complete table 2.(4 marks)
Calculate the;I
II
III
Final burette reading(cm3)
Initial burette reading(cm3)
Volume of solution B used (cm3)
- Average volume of solution B used. (1 marks)
- Number of moles of potassium manganate (VII) used. (1 mark)
- Number of moles of A in 25cm3 of solution A given that 2 moles of Potassium manganate (VII) reacts completely with 5 moles of A. (1 mark)
- Calculate the concentration of solution A in moles/ litre (1mark)
- Relative formula mass of A. (2 marks)
- The formula of A has the form H2C2O4•xH2O. Determine the value of x in the formula given that the relative mass of carbon is 12 and that of oxygen and hydrogen are 16.0 and 1.0 respectively. (2 marks)
- You are provided with solid P. Carry out the tests below. Write your observations and inferences in the spaces provided. Add 10cm3 of water to the solid P, shake well and filter the mixture.
Use the filtrate for tests (a)(i) to (iii)
Retain the residue for test (b)
Observations
Inferences
(1 mark)
(1 mark)
(a) i) To about 2cm3 of filtrate add 2cm3 of NaOH solution, warm the mixture and test anygases produced using blue and red litmus papers.
|
Observations |
Inferences |
|
(1 mark) |
(1/2 mark) |
ii) To about 2cm3 of filtrate add 3drops of Lead(II)nitrate solution
|
Observations |
Inferences |
|
(1 mark) |
(1 mark) |
To about 2cm3of filtrate add 3 drops of acidified Barium nitrate solution.
|
Observations |
Inferences |
|
(1/2mark) |
(1/2 mark) |
(b) To the residue add HNO3 drop wise until the solid dissolves. Test any gases produced using a burning wooden splint.
|
Observations |
Inferences |
|
(1 mark) |
(1/2 mark) |
To about 2cm3 of solution add Ammonia solution dropwise until in excess
|
Observations |
Inferences |
|
(1 mark) |
(1 mark) |
To about 2cm3 of solution add NaOH dropwise until in excess.
|
Observations |
Inferences |
|
(1 mark) |
(1/2mark) |
To about 2cm3 of solution add 3 drops of Potassium iodide solution
|
Observation (1mk ) |
Inferences (1/2mk) |
|
Observations |
Inferences |
|
( ½ mark) |
( ½ mark) |
- You are provided with solution Q. carry out the following tests and record your observations and inferences in the spaces provided. Divide the solution into 5 portions
(a) To the 1st portion add 2cm3 of Distilled water.
(b) To 2nd portion add 2 to 3 drops of acidified potassium Manganate(VII) solution
|
Observations |
Inferences |
|
(1/2mk) |
(1mk) |
(c) To 3rd portion, add 2 to 3 drops of Bromine water
|
Observations |
Inferences |
|
(1/2mk) |
(1mk) |
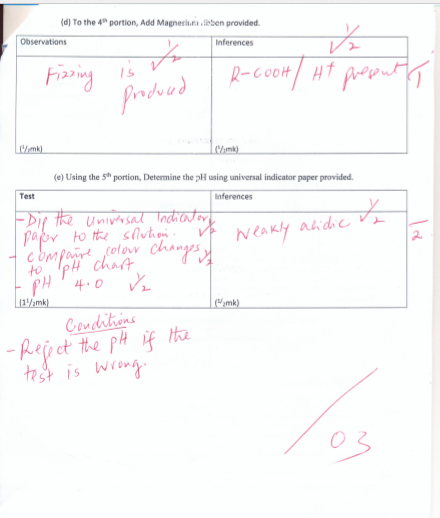
(d) To the 4th portion, Add Magnesium ribbon provided.
|
Observations |
Inferences |
|
(1/2mk) |
(1/2mk) |
(e) Using the 5th portion, Determine the pH using universal indicator paper provided.
|
Test |
Inferences |
|
(11/2mk) |
(1/2mk) |

MARKING SCHEME
Download CHEMISTRY PAPER 3 - 2019 MOKASA II MOCK EXAMINATION.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students