233/1
CHEMISTRY
PAPER 1
TIME: 2 HOURS
INSTRUCTIONS TO CANDIDATES
- Answer all questions in the spaces provided
- KNEC mathematical tables and silent electronic calculators may be used
- All workings must be clearly shown where necessary
- Candidates should answer all questions in ENGLISH
QUESTIONS
-
- What is meant by allotropy? (1mk)
- Identify the two crystalline allotropes of carbon. (1mk)
- Give one use of carbon black. (1mk)
- When hydrated sample of iron (II) Sulphate FeSO4.nH2O was heated until there was no further change in mass, the following data was recorded.
Mass of evaporating dish = 78.94g
Mass of evaporating dish + hydrated salt = 84.14g
Mass of evaporating dish + residue = 81.78g
Determine the empirical formula of the hydrated salt
(Relative formula Mass of FeSO4 = 152, H2O =18) (3mks) - Equal volumes of 2M monobasic acids R and S were each reacted with excess magnesium ribbon. The table below shows the volume of the gas produced after one minutes
Acid
Volume of gas (cm3)
R
80
S
30
- Write the ionic equation for reaction which took place (1mk)
- Explain the difference in the volumes of the gas produced (2mks)
- The graph below shows the changes which takes place when a solid is heated.
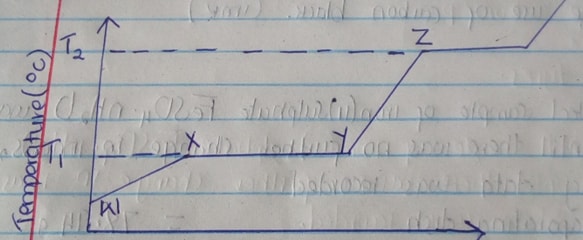
- What happened to the molecules between W and X? (1mk)
- What is the significance of temperatures T1 and T2 (1mk)
- Explain why the temperature does not rise between X and Y (1mk)
- In an experiment to determine the solubility of potassium nitrate at 300c, a saturated solution was heated in an evaporating dish until there was no further change in mass. The following data was obtained.
Mass of dish + solution = 128.9 g
Mass of dish + dry salt = 103.9 g
Mass of empty dish = 94.3 g
Determine the solubility of potassium nitrate at 300c. (3mks) - The diagram below shows a set up that was used to prepare and collect a sample of nitric acid.
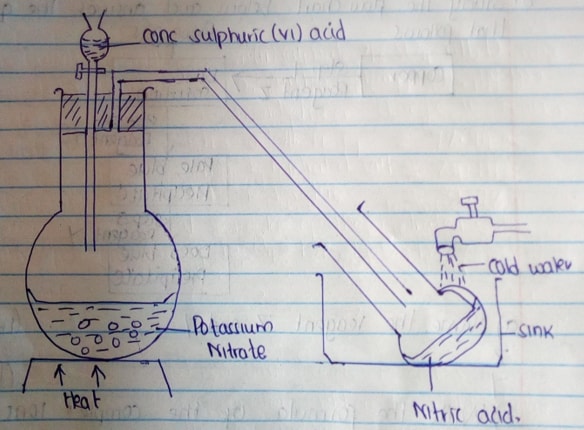
- Give a reason why it is possible to separate nitric acid from Sulphuric acid in the set up. (1mk)
- Name another substance that can be used instead of potassium nitrate. (1mk)
- Starting with lead oxide, nitric acid, sodium sulphate, water and all necessary apparatus, describe how you would prepare a dry sample of lead (II) sulphate (3mks)
- Study the flow chart below and answer the questions that follows:
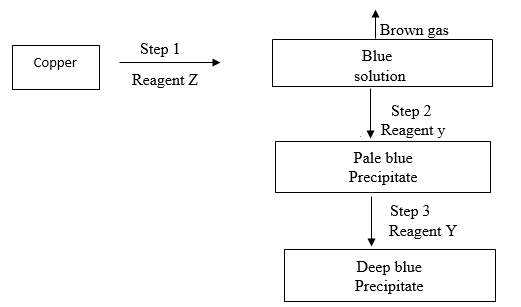
- Name the reagent Z and Y
Z (1mk)
Y (1mk) - Write the formula of the complex ions presented in the deep blue solution (1mk)
- Name the reagent Z and Y
- The equations below shows the molar enthalpies of combustion of carbon, hydrogen and methane.
C(s) + O2(g) → CO2 (g) ΔHc = -393 KJmol-1
H2 (g) + ½ O2 (g) → H2O (l) ΔHc = -285 KJmol-1
CH4 (g) + O2 (g) → CO2 (g) ΔHc = -890KJmol-1
Use the energy cycle diagram to calculate the heat of formation of methane (3mks) - NO2 and N2O4 gases exist in equilibrium at 200c
NO2(g) ⇌ N2O4(g) ΔH = -ve
State and explain the observation that would be made when- A syringe containing the mixture 200c is heated to 400c (1mk)
- The gaseous mixture in a syringe is compressed. (1mk)
- The diagram below shows a Bunsen burner when in use
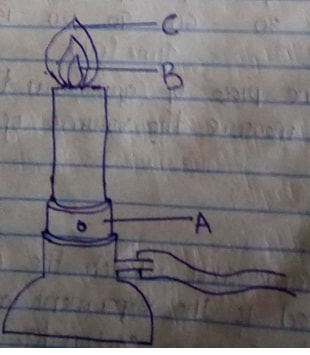
- Name the regions labelled B and C (1mk)
- What is the function of the part labelled A? (1mk)
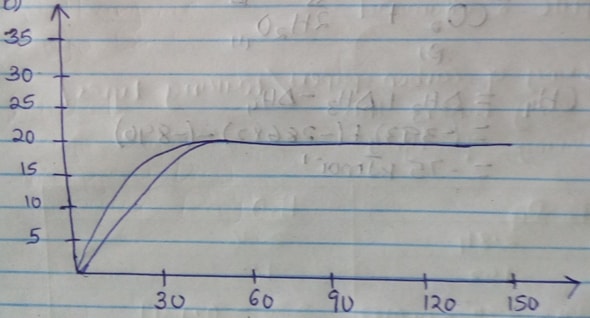
- A certain mass of marble chips reacted with excess dilute hydrochloric acid at 250c. The volume of carbon (IV) oxide gas liberated was measured after 30 seconds. The results were presented as shown in the graph below.
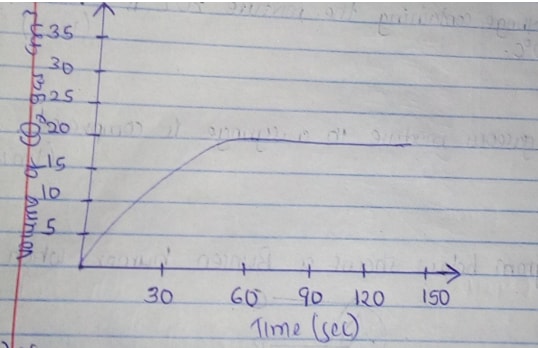
- Name one piece of apparatus that may have been used to measure the volume of the gas liberated. (1mk)
- On the same axis sketch the curve that would be obtained if the experiment was repeated using powdered calcium carbonate. (1mk)
- When hydrogen Sulphide gas was bubbled into an aqueous solution of iron (iii) chloride, a yellow precipitate was deposited.
- State another observation that would be made (1mk)
- Write an equation of the reaction that took place. (1mk)
- The table below shows the atomic number of elements M, P, Q and R.
Element
P
Q
R
M
Atomic No
13
7
12
13
Mass No
26
15
24
27
- Which two letters represent the same element? Give reasons (1mk)
- Give the number of neutrons of an atom of element Q (1mk)
- The diagram below show the set up that was used to prepare and collect sulphur (IV) oxide gas.
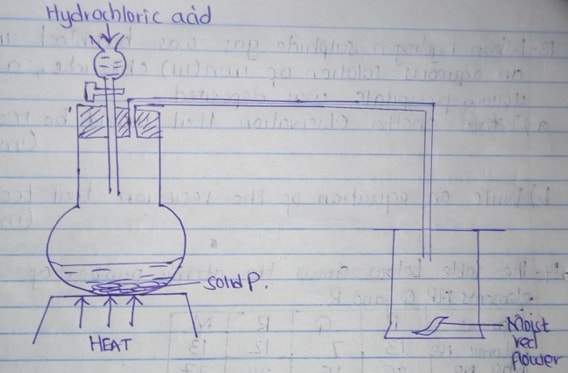
- Identify the solid P (1mk)
-
- Why is it possible to collect Sulphur (iv) oxide as shown? (1mk)
- What happened to the red flower? (1mk)
-
- State Charles’ law (1mk)
- The volume o f a sample of nitrogen gas at temperature of 298k and 600mmHg pressure was 0.048 m3, calculate the temperature at which the volume of the gas would be 0.032 m3 if pressure remains the same. (2mks)
- Element T consists of two isotopes 62T and 64T in the ratio 7:3 respectively. Calculate the Relative atomic mass of element T (3mks)
- Name the process which takes place when
- Solid carbon (iv) oxide changes directly into gas (1mk)
- Butanol reacts with hexanoic acid in the presence of Sulphuric (iv) acid. (1mk)
- Study the standard electrode potentials for the half-cells give below and answer the questions that follows ( the letters do not represent the actual symbols of the elements)
Eθ volts
N+(aq) + e− → N(s) − 2.92
J+(aq) + e− → J(s) + 0.52
K+(aq) + e− → K(s) − 0.00
G+(aq) + e− → G(s) + 1.36
M+(aq) + 2e− → M(s) − 0.44- Identify
- The strongest reducing agent (½ mks)
- The strongest oxidizing agent (½mks)
- Calculate the e.m.f of the cell (2mks)
N(s)/N+(aq) // G+(aq) / G(s)
- Identify
- Study the table below and answer the questions that follow
Bond type
Bond energy (KJ/mol)
C - C
346
C = C
610
C - H
413
C - Br
280
Br - Br
193
- Calculate the enthalpy of the following reaction. (2mks)
C2H4(g) + Br2(g) → C2H4Br2 (g) - Name the type of reaction that took place in a) above (1mk)
- Calculate the enthalpy of the following reaction. (2mks)
- Briefly explain how you would obtain pure sample of lead (ii) chloride from a mixture of lead (ii) chloride and silver chloride (3mks)
- Explain the following observations: very little carbon (iv) oxide is evolved when lead carbonate reacts with dilute hydrochloric acid (2mks)
- The table below gives some properties of compounds P, Q, R and S
Compound
B.P0C
M.P0C
Conductivity in water
P
77
-23
Does not conduct
Q
74
-19
Does not conduct
R
-161
-85
Conduct
S
2407
714
Conduct
- Which one of the compounds in the table is ionic?
Explain (1mk) - Give the compound that is liquid at room temperature. (1mk)
- Which one of the compounds in the table is ionic?
- When butan–1–ol is oxidized by acidic potassium dichromate, a weak organic acid is formed. Draw and name the structure formula of the acid obtained from the above reaction. (2mks)
- When a hydrocarbon fuel burns, one of the main products is acidic gas R
- Identify gas R (1mk)
- What two effects does gas R have when its concentration in the atmosphere exceeds its acceptable level. (2mks)
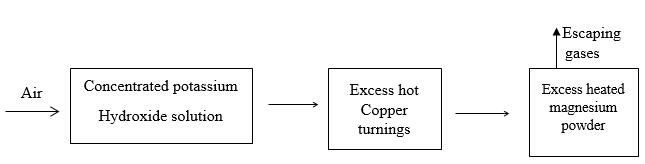
- Air was passed through several reagents as shown in the flow chart below.
- Write an equation for the reaction that took place in the chamber with the magnesium powder (1mk)
- Name one gas that escapes from the chamber containing magnesium powder. Give a reason for your answer. (1mk)
- When a current of 6.42 Amperes was passed through an electrolyte Y2+ for 10 minutes, 2.74g of Y were deposited. (1mk)
- Calculate the quantity of the electricity passed in the experiment.
- Determine the relative atomic mass of (1 faraday = 96,500 coulombs) (2mks)
- Explain why aluminium metal is not extracted from aluminium chloride (2mks)
- Part of the structure of a polymer is given below.
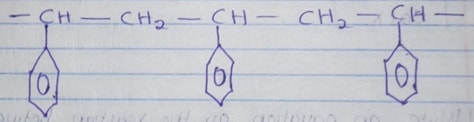
- Identify the polymer. (1mk)
- State one disadvantage of continued use of this polymer (1mk)
- The table below gives the rate of decay for a radioactive element M
Number of days
Mass (g)
0
12.8
280
0.8
Determine the half – life of the radioactive element M (2mks) - Study the flow chart below and answer the questions that follows.
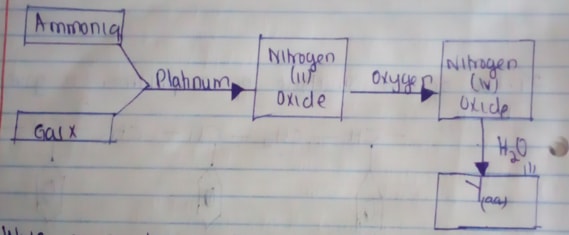
- Write an equation for the reaction between gas X and ammonia (1mk)
- Write the formulae of the substance present in the mixture Y(aq) (2mks)
- When the air hole is fully opened, the Bunsen burner produces a non-luminous flame
Explain (1mk)

MARKING SCHEME
-
- Existence of element in more than one physical form in the same state
- Graphite, diamond
- Making of carbon papers / making tyres/ making printers ink
-
FeSO4 H2O Mass 2.84 2.36 RFM 152 18 No of moles 2.84 = 0.0187
1.522.36 = 0.1311
18Mole ratio = 0.0187 = 1
0.01870.1311 = 7
0.0187
E.F = FeSO4.7H2O -
- Mg(s) + 2H+(aq) → Mg2+ (aq) + H2(g)
- Acid R is stronger than acid S
Acid S is stronger acid while S is weak acid
It produces more H+ ions which react with magnesium
It ionizes fully in water or it produces high volume of hydrogen.
-
- They gain K.E
They gain energy and vibrate faster - T1 – Melting point
T2 – Boiling point - Energy is used to weaken the intermolecular force of attraction so as to change the substance from solid to liquid state.
- They gain K.E
- Mass of solution = 128.9 - 94.3 = 34.6 (g)
Mass of dry salt = 103.9 -94.3 =9.6 (g)
Mass of solvent = 34.6 – 9.6 = 25 (g)
25.0g of solvent containing 9.6g
100g =?
9.6 x 100
25
Solubility = 38.4g/100g of water -
- Nitric (v) acid is more volatile than conc sulphuric (vi) acid or
Nitric (v) acid has a lower B.P than Sulphuric (vi) acid. - Sodium nitrate
- Nitric (v) acid is more volatile than conc sulphuric (vi) acid or
- React excess lead oxide with the nitric acid filter to form lead nitrate solution. Dissolve sodium sulphate in water to form solution. Mix sodium sulphate solution with lead nitrate solution to precipitate lead sulphate. Filter, wash the residue to dry between filter paper.
-
- Z – concentrated nitric (v) acid
Y – Ammonia solution / ammonium hydroxide, aqueous ammonia. - (CU (NH3)4)2+
- Z – concentrated nitric (v) acid
-
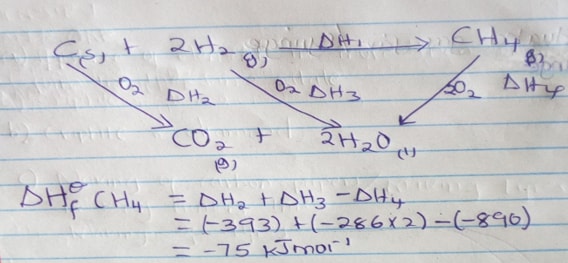
-
- Brown colour intensifies, Reaction is exothermic
Increasing the heat will favour backward reaction
Equilibrium shift to the left and this reaction absorbs heat - Pale yellow colour intensifies
Equilibrium shifts to the right because volume is reduced
- Brown colour intensifies, Reaction is exothermic
-
- B – Unburnt gas/colourless region/almost colourless region
C – Pale blue region - Regulating amount of air entering the chimney
- B – Unburnt gas/colourless region/almost colourless region
-
- Graduated gas jar/syringe
-
-
- The solution turned from yellow to pale green
Red brown to pale green/ brown to pale green - 2FeCl3(aq) + H2S(g) → 2FeCl2(aq)+S(s) +2HCl(aq)
- The solution turned from yellow to pale green
-
- P and M, They have same atomic number
- n = 15 – 7
= 8
-
- Identify the solid P
Sodium Sulphite/Potassium Sulphite -
- Its denser than air /it was bleached/ it turned white.
- Remained red
- Identify the solid P
-
- The volume of a fixed mass of gas is directly proportional to its absolute temperature at constant pressure.
- V1 = V2 0.048 = 0.032 T2 = 198.667 K
T1 T2 298 T2
- R.A.M = 7 × 62 + 3 × 64
10 10
= 43.4 + 19.2
= 62.6 -
- Sublimation
- Esterification
-
-
- N
- G
- E.M.F cell = E reduction – E oxidation
= (+ 1.36) – (-2.92)
= + 4.29v
-
-
- Bond breaking
(C = C) + (Br – Br) + 4 (C – H)
+ 610 KJmol-1 + 193 KJmol-1 + 1652 KJmol-1
= 2455 KJmol-1
Bond formation
2 (C – Br) + (C – C) + 4 (C – H)
560 + 346 + 1652
= 2558
Heat of reaction = Bond breaking + Bond formation
= 2455 KJmol-1 + (- 2558 KJmol-1)
= -103KJmol - Addition reaction/Halogenation/Exothermic/Bromination
- Bond breaking
- Add warm water to the mixture and stir
PbCl2 dissolves while silver chloride does not
Filter to obtain lead(II) chloride as filtrate and silver chloride as residue.
Cool the filtrate to obtain solid lead (II) chloride - Lead (II) carbonate react with dilute hydrochloric acid to form an insoluble coat of lead (II) chloride on the carbon which stops further reaction
-
- S because it has a high M.P and B.P and also conducts in aqueous solution
- P or Q
-
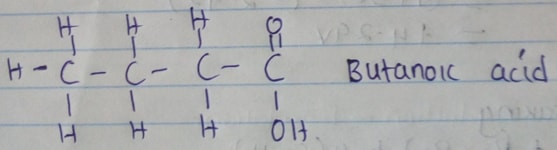
-
- Carbon (iv) oxide/CO2
- Leads to global warming/greenhouse effect/acid rain
-
- 3Mg(s) + N2(g) → Mg3N2(g)
- Neon/Argon, it is noble gas
-
- Quantity of = 1t
Electricity = 6.42 x 10 x 60
= 6.42 x 600
= 3.852C - 3852c produce 2.74g
2 x 96500 = 2 x 96500 x 2.74
3852
= 137.28
- Quantity of = 1t
- AlCl3 is largely covalent /it sublimes when heated
It is made of molecules which do not conduct electricity. -
- Polyphenylethane/polystyrene
- It is non-biodegradable/pollutes environment
-
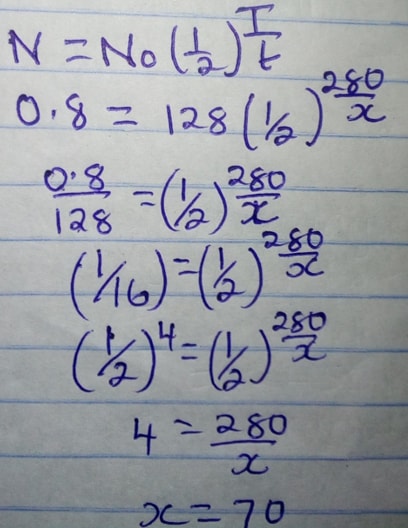
12.8(g) — 6.4g — 3.2g — 1.6g — 0.8g
4 t½ = 280 days
t½ = 280
4
= 70 days -
- 4NH3(aq) + 5O2(g) → 4NO(g) + 6H2O(l)
- HNO3 (aq) and HNO2 (aq)
- The laboratory gas burns in excess oxygen / burns completely/produces CO2 and H2O only/No unburnt carbon remains/No soot is produced.
Download Chemistry Paper 1 Questions and Answers - Murang'a County Mocks 2020/2021.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students