233/2
CHEMISTRY PAPER 2
TIME: 2 HOURS
INSTRUCTIONS TO CANDIDATES
- Answer all the questions in the spaces provided
- KNEC mathematical tables and silent electronic calculators may be used
- All workings must be clearly shown where necessary
- Candidates should answer all questions in ENGLISH

QUESTIONS
- A student set-up the following apparatus to prepare carbon (II) oxide from charcoal in the laboratory.
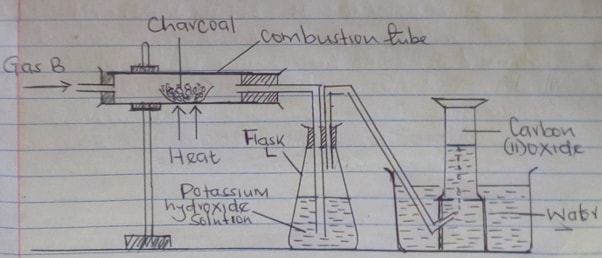
- State the purpose of potassium hydroxide solution (1mk)
- Identify gas B (1mk)
- Name two substances that react together to produce gas B (2mks)
- Write balanced equations for reactions in
- Combustion tube (1mk)
- Flask L (1mk)
- Describe two simple test that you would use to distinguish between Carbon (IV) oxide and Carbon (II) oxide. (2mks)
- In another experiment, the student reacted charcoal with excess hot concentrated nitric (V) acid.
- State one observation made (1mk)
- Write balanced equation for the reaction (1mk)
- State two use of Carbon (II) oxide (1mk)
- Use the information in the table below to answer the questions that follow. The letters are not the actual symbols of the elements.
Element Atomic Number M.P (oC) A 11 97.8 B 13 660 C 14 1410 D 17 −95 E 20 839 - Write the electronic arrangement for the ions formed by elements D and A (2mks)
- Select an element which is:
- A poor conductor of electric current (1mk)
- The strongest reducing agent (1mk)
- Has a giant covalent structure (1mk)
- In which state will element B exists at 661oc Explain. (1mk)
- Compare the electrical conductivity of element A and B. Give a reason (1mk)
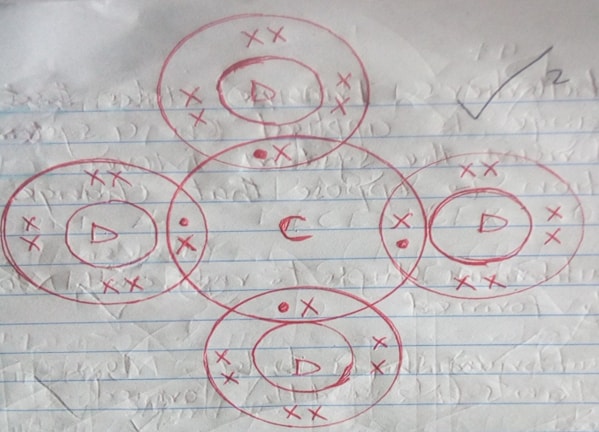
- Using dots (.) and crosses (x) to represent the outermost electrons, show the bonding in the compound formed between elements C and D. (2mks)
- Explain the difference in melting points in elements B and A (2mks)
- Write an equation for the reaction that takes place between element E and steam. (1mk)
- Describe how a solid mixture of the Chloride of E and lead (II) Sulphate can be separated into solid sample. (2mks)
- Study the flow chart below and answer the questions that follow.
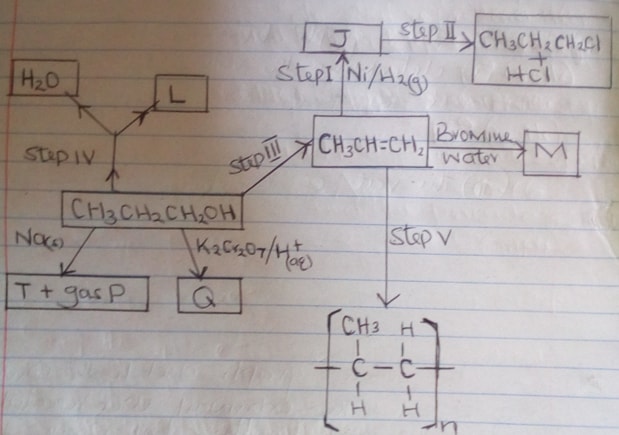
- Name substance J and draw its structural formula: (2mks)
Name
Structural formula - What reagents and conditions are necessary for:
- Step (III) : Reagent (1mk)
Condition - Step II: Reagent (1mk)
Condition
- Step (III) : Reagent (1mk)
- Name the following
- L (1mk)
- Gas P (1mk)
- Q (1mk)
- M (1mk)
- Write the equation of the reaction that occur in step (IV) (1mk)
- Give the name of process in step (V) (1mk)
- If the relative Molecular Mass of R is 21,000, determine the value of n. (C = 12.0, H = 1.0) (2mks)
- Name substance J and draw its structural formula: (2mks)
-
- Define an electrolyte (1mk)
- Explain why the following substances conduct an electric current (2mks)
- Magnesium metal
- Molten magnesium Chloride
- Study the reaction scheme below and answer the questions that follow.
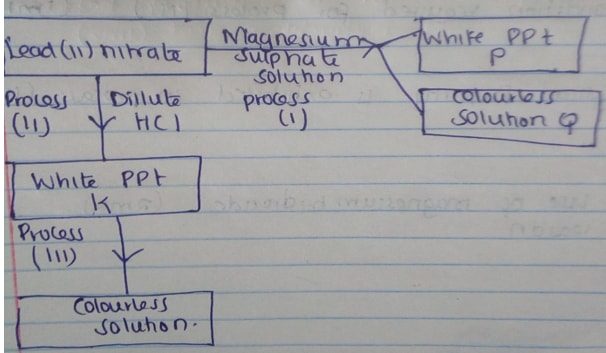
- Write the formula of P and Q (2mks)
- Write an ionic equation for the formation of P (1mk)
- Name process (i) (1mk)
- Write a balanced equation for the formation of white precipitate K (1mk)
- State the condition required for process (III) (1mk)
- Which physical property is exhibited in process (III) (1mk)
- State one use of magnesium hydroxide (2mks)
Give one reason
-
- At 250c, 50g of potassium nitrate were added to 100g of water to make a saturated solution. What is meant by a saturated solution? (1mk)
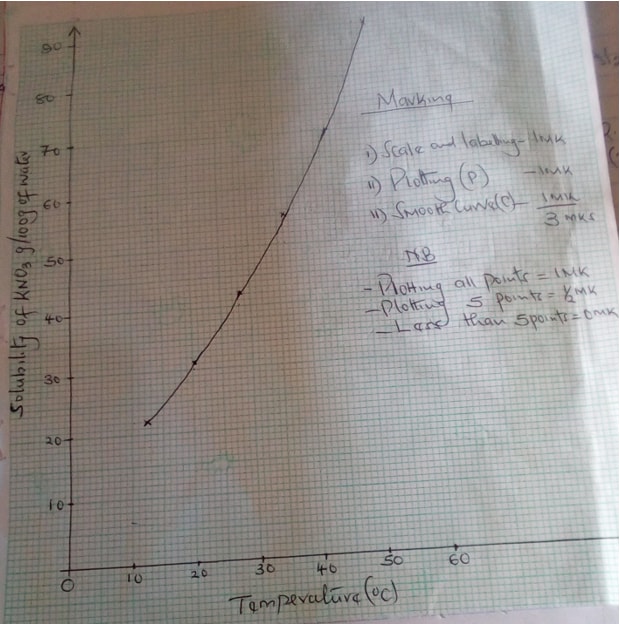
- The table below gives the solubilities of potassium nitrate at different temperatures.
Temperature (oC) 12 20 28 36 44 52 Solubility g/100g of water 22 31 42 55 70 90 - Plot a graph of the solubility of potassium nitrate (vertical axis) against temperature (3mks)
- Using the graph
- Determine the solubility of potassium nitrate at 150c. (1mk)
- Determine the mass of potassium nitrate that remained undissolved given that 80g of potassium nitrate were added to 100cm3 of water and water to 400c. (2mks)
- Plot a graph of the solubility of potassium nitrate (vertical axis) against temperature (3mks)
- Determine the molar Concentration of potassium nitrate at 150c. (Assume there is no change in density of water at this temperature) (K = 39.0, N = 14.0, O = 16.0) (3mks)
-
- Aluminium oxide reacts with both acids and bases
- Write an equation for the reaction between aluminium oxide and hydrochloric acid (1mk)
- Using the equation in (a) above, calculate the number of moles of hydrochloric acid that would react completely with 153.0g of aluminium oxide (Al = 27.0, O = 16.0) (3mks)
- Sodium hydroxide pellet were accidentally mixed with sodium chloride, 8.8g of the mixture were dissolved in water to make one litre of solution. 50cm3 of the solution was neutralized by 20.0cm3 of 0.25M Sulphuric (VI) acid.
- Write the equation for the reaction that took place. (1mk)
- Calculate the:
- Number of moles of the substance that reacted with Sulphuric (VI) acid (2mks)
- Number of moles of the substance that would react with Sulphuric (VI) acid in the one litre solution. (1mk)
- The percentage of sodium chloride in the mixture. (2mks)
- Aluminium oxide reacts with both acids and bases
- The flow chart below illustrates the industrial extraction of lead metal.
Study it and answer the questions that follow.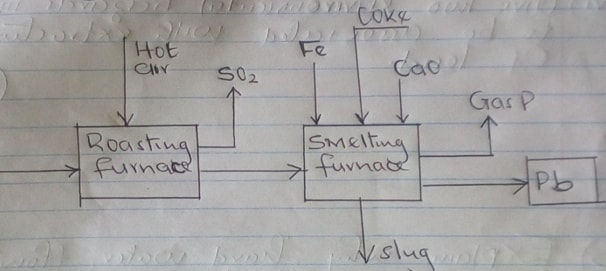
-
- Name the ore that is commonly used in the process (1mk)
- Explain what takes place in the roasting furnace (1mk)
- Identify gas P (1mk)
- Write the equation for the main reaction that takes place in the smelting furnace. (1mk)
- What is the purpose of adding iron in the smelting furnace? (1mk)
- Give two environmental hazards likely to be associated with extraction of lead. (2mks)
- Explain why hard water flowing in lead pipes may be safer for drinking than soft water flowing in the same. (2mks)
- State one use of lead other than the making of lead pipes (1mk)
-

MARKING SCHEME
-
- To absorb excess Carbon (IV) oxide or gas B
To absorb unreacted Carbon (IV) oxide any one - Carbon (IV) oxide / CO2
- Any Carbonate/hydrogen Carbonate and acid
-
- CO2 (g) + C(s) → 2CO (g)
- KOH (aq) + CO2 (g) → KHCO3 (aq)
- Use of Ca(OH)2(aq): CO does not form white precipitate with Ca(OH)2 while CO2 does CO burns with a blue flame while CO2 does not support combustion.
-
- Brown fumes produced
Black substance dissolves any one - HNO3 (aq) + C(s) → CO2 (g) + 4NO2 (g)
- Brown fumes produced
- Reducing agent in the extraction of metals from the ores
Used as fuels any one correct
Manufacture of hydrocarbons
- To absorb excess Carbon (IV) oxide or gas B
-
- 2.8.8, 2.8
-
- D
- A
- C
- Liquid - The melting point is below 6610c
- B is a better electric conductor than A, because it has more delocalized electrons than A.
-
- B has a higher melting point than A because B has more delocalized electrons than A.
Therefore B has stronger metallic bond than A thus high melting point. - E(s) + H2O (g) → EO(s) + H2 (g)
- Add water to the mixture, Stir, E Chloride dissolves while Lead (II) Sulphate does not. Filter and wash the residue with distilled water.
Evaporate the filtrate to obtain solid E Chloride
-
- Propane
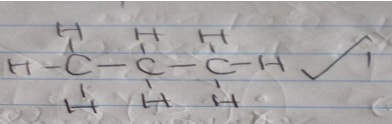
-
- Reagent – Conc Sulphuric (VI) acid/Conc H2SO4
Condition – 160 – 1800c - Reagent: Chlorine gas/C/(g)
Condition: Ur light/sunlight
- Reagent – Conc Sulphuric (VI) acid/Conc H2SO4
-
- Carbon (IV) oxide
- Hydrogen gas
- Propan-1-oic acid
- 1-Bromopropane / 2 – Bromopropane
- 2C3H7OH (l) + 9O2 (g) → 6CO2 (g) + 8H2O (l)
- Addition polymerization / polymerization
- (3 x 12) + (1 x 6) = 42
42n = 21,000
N = 21,000 = 500 units
42
- Propane
-
- Are substances which when molten or dissolved in water conduct an electric current and decomposes.
-
- Magnesium metal conducts since it contains free electrons
- Molten magnesium Chloride conducts since it contains free ions
-
- P – PbSO4
Q – Mg (NO3)2 - Pb2+(aq) +SO42- (aq) → PbSO4 (s)
- Precipitation / Double decomposition
- Pb(NO3) (aq) + 2HCl (aq) → PbCl2(s) + 2HNO3 (aq)
- Heat/warm
- Effect of temperature on solubility
- It is used as anti-acid medicine because Mg (OH) 2 is a non-toxic base.
- P – PbSO4
-
- A solution which cannot dissolve any more solute at a particular temperature.
-
-
-
- 25g per 100g of water
- Mass dissolved = 62g
Mass of undissolved = 80 – 62 = 18g
-
- R.F.M of KNO3 =101
Moles of KNO3 in 100g water = 25 = 0.2475 moles
101
Moles in 1000g of water = 0.2475 x 1000
100
= 2.475 moles
-
-
- Al2O3(s) + 6HCl (aq) → 2AlCl3 (aq) + 3 H2O (l)
- R.F.M of Al2O3
(27 x 2) + (16 x 3) = 102
Moles of Al2O3 = 153 = 1.5 moles
102
Moles of HCl = 1.5 x 6 = 9 moles
-
- 2NaOH (aq) + H2SO4 (aq) → Na2SO4 (aq) + 2H2O (l)
-
- Mole ratio of NaOH: H2SO4 = 2: 1
Moles of H2SO4 reacted = 20 x 0.25 = 0.005 moles
1000
Moles of NaOH reacted = 2 × 0.005 = 0.01moles - If 50cm3 of NaOH = 0.01 moles
1000cm3 of NaOH = 1000 × 0.01
50
= 0.2 moles - Molar mass of NaOH = 40 gmol-1
Mass of NaOH reacted = 40 x 0.2 = 8g
Mass of NaCI = 8.8 – 8.0 = 0.8g
% of NaCI = 0.8 x 100 = 9.090%
8.8
- Mole ratio of NaOH: H2SO4 = 2: 1
-
-
-
- Galena
- Some of the Sulphide is converted into Lead Oxide and Sulphur (IV) oxide
- Carbon (II) oxide or carbon (IV) oxide
- PbO(l) + C (s) → Pb (l) + CO (g)
- To reduce unreacted Pbs to Pb
- Sulphur (IV) oxide – causes acid rain
Lead – causes lead poisoning
- Hard water contains Mg2+/Ca2+ ions. These ions form a protective layer of calcium Sulphate or Magnesium Carbonate hence does not dissolve lead.
Soft water does not form these deposits. - Radioactive shielding
Lead acid accumulators
Making roof
-
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 2 Questions and Answers - Murang'a County Mocks 2020/2021.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

