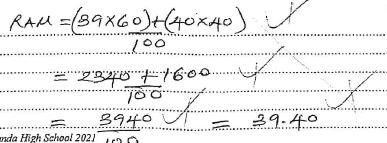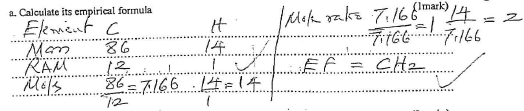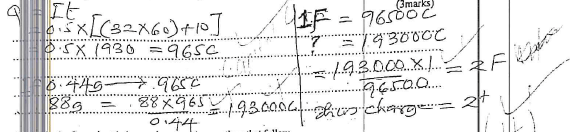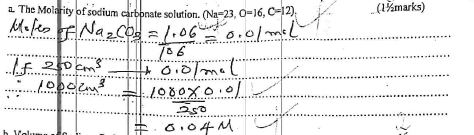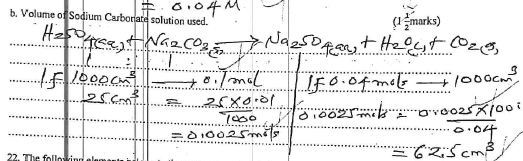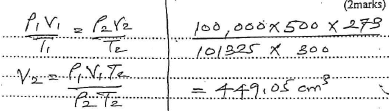- State the process that takes place when; (3marks)
- Natural fats or oil are hydrolysed using alkalis
- Sulphur is added to natural-rubber-and-heated to form cross links
- A heavy nuclide is broken down by a fast-moving neutron.
-
- Define the term homologous series as used in the study of organic chemistry. (1mark)
- Draw the structural formula of the third member of alkyne homologous series and name it. (2marks)
- The element X has isotopic species A and B each with a mass number 39 and 40 respectively. The percentage of A is 60% and in B is 40% for an isotopic element X. Calculate the relative atomic mass of X. (2marks)
-
- Define the term solubility (1 mark)
- 70 g of salt W were added to 80 cm3 of water at 25°C. After stirring 10g of crystals of salt W were filtered out. Determine the solubility of salt W at 25°C (2marks)
-
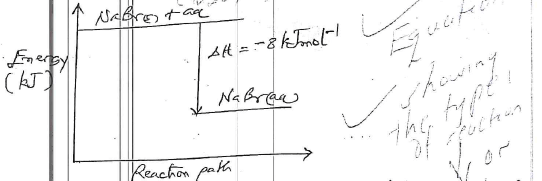
- Define molar heat of solution (1mark)
- The lattice energy of 500mm bromide and hydration energies of sodium and bromide ions are 733, 406 and 335Kjmol' respectively.
- Determine the heat of solution of Sodium Bromide (2marks)
- Sketch an energy level diagram of the process (2marks)
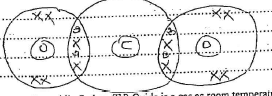
- The table below gives some experimental results on four samples of water. Use the information to answer the questions that follow.
Drops of soap needed for form lather Sample of water ( 50 cm3) Before boiling After boiling A 25 15 B 8 8 C 18 8 D 25 25 - Which samples contain permanent hardness? Explain (11/2 marks)
- Which samples are likely to contain calcium hydrogen carbonate? Explain. (11/2 marks)
- Describe how you could prepare dry sample of Lead (II) Chloride starting with Lead (II) Carbonate. (3marks)
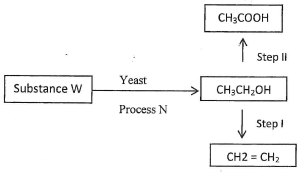
- Study the flow chart below and use it to answer question that follow.
- Name the process N(1mark)
- Suggest a suitable reagent for use in step II (1mark)
- Describe how step I is carried out. (1mark)
- Sodium hydroxide solution and graphite are both capable of conducting and electric current. What is the major difference between the two substances in their electrical conductivity? (1 mark)
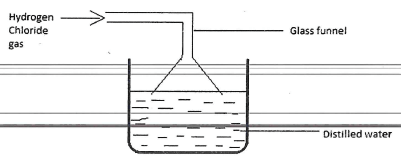
- An aqueous solution of hydrogen chloride can be prepared as shown in the diagram below.
- Give two reasons for using an inverted funnel (1mark)
- Four drops of Lead (II) Nitrate solution were added to 4cm3 of the solution obtained above followed by excess aqueous ammonia. Explain the observation. (2marks)
- Nitrogen reacts with oxygen according to the equation.
N2 (g) + O2(g) ⇌ 2NO(g), ΔH = +197kJmol-1.
What is the effect of increase of the following on the position of the equilibrium? Explain.- Pressure (11/2 marks)
- Temperature (11/2marks)
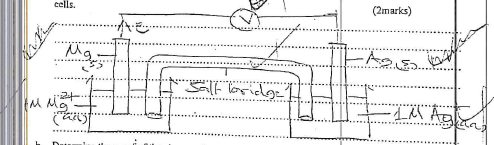
- The standard reduction potentials of two half cells are
Ag+(aq) + e- → Ag(s) Eθ = +0.80V
Mg2+ (aq) + 2e- → Mg(s); Eθ=-2.37v- Draw a well labelled diagram of an electrochemical cell that can be constructed using the two half cells. (2marks)
- Determine the c.m.f of the above cell (1 mark)
- A white solid L changes to yellow solid R when strongly heated but on cooling becomes white again when L is reacted with dilute nitric(v)acid effervescence occurs.
- Identify L (1 mark)
- Write the equation for the reaction of L and Nitric (v) acid. (1 mark)
- Name and write the formula of the salt formed when solid R dissolves in Sodium Hydroxide solution. (1 mark)
- The table below shows the pH values of J to N
Solution J K L M N pH 5 13 2 10 1 - Which solution
- Contains the largest concentration of hydroxide ions? Explain (1 mark)
- is likely to be a solution of ethanoic acid? Explain (1 mark)
- Which solution
-
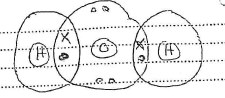
- Using dots (0) and crosses (x), show the bonding in; (2marks)
- Water
- Carbon(IV)Oxide
- Why is water a liquid at room temperature while Carbon (IV) Oxide is a gas as room temperature? (1 mark)
- Using dots (0) and crosses (x), show the bonding in; (2marks)
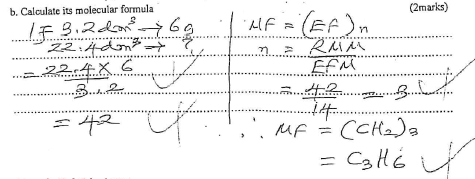
- A gaseous compound consists of 86% carbon and 14% hydrogen by mass. At s.t.p 3.2 dm3 of the compound has a mass 6g. (C=12; H=1, Molar gas volume at s.t.p-22.4dm3)
- Calculate its empirical formula (1 mark)
- Calculate its molecular formula (2 marks)
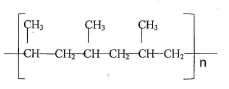
- The structure below represents a segment of a polymer. Use it to answer the questions that follow.
- Draw the structure of the monomer (1 mark)
- Given that the molecular mass of the polymer is 2,000 find the value of n (2marks)
- The chief are aluminum bauxite which mainly contains AL203.2H2O. The ore is initially purified before aluminum is extracted electronically.
- Identify the main impurities associated with this ore. (1 mark)
- Sodium hydroxide solution is used in the purification process. State its role. (1 mark)
- Give an equation for the reaction that forms aluminum oxide (Alumina) from Aluminum hydroxide. (1 mark)
- When a current of 0.5 amperes was passed for 32 minutes and 10 seconds through chloride of metal P. 0.44g of P was deposited. Determine the charge of the ion of metal P. (1f=96500c, p=88) (3marks)
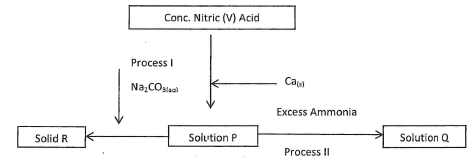
- Study the flow chart below and answer the questions that follow.
- Identify the solution P (1 mark)
- Write the chemical equation to show how solid R is formed. (1 mark)
- State the observation made in process II (1 mark)
- If 25.0 cm3 of 0.1M H2SO4 solution neutralized a solution containing 1.06g of sodium carbonate in 250 cm3 of solution. Calculate,
- The Molarity of sodium carbonate solution. (Na=23, O=16, C=12) (11/2 marks)
- Volume of Sodium Carbonate solution used. (11/2 marks)
- The following elements belong to the same group of periodic table. (Letters do not represent the actual symbols)
Element Atomic radius (nm) Ionic radius (nm) 1st Ionization Energy (kJmol-1) P 0.137 0.066 736 Q 0.089 0.031 900 R 0.174 0.099 590 - State whether the elements are metals or non-metals. Explain your answer. (2marks)
- Which element is the most reactive? Explain (2marks)
- Given the following substances: wood ash, lemon, juice and sodium chloride.
- Name one commercial indicator that can be used to show whether wood ash, lemon juice and sodium chloride and acidic, basic and neutral (1 mark)
- Classify the substances in 23(a) above as acid, base and neutral (2 marks)
Acid Base Neutral
- A compound whose general formula is M(OH)3 reacts as shown by the equation.
M(OH)3(s) + OH-(aq) → M(OH)4-(aq)
M(OH)3(s) + 3H+(aq) → M3+(aq) + 3H2O(l)- What name is given to compounds which behave like M(OH)3 in the two reactions. (1 mark)
- Name two elements whose hydroxides behave like that of M. (2 marks)
- In an experiment on rates of reaction, Potassium Carbonate was reacted with dilute Sulphuric(VI) acid.
- What would be the effect of an increase in the concentration of the acid on the rate of reaction? (1 mark)
- Explain why the rate of reaction is found to increase with temperature. (2 marks)
-
- State Boyles law. (1mark)
- A gas occupies 500cm3 at 27°C and 100,000Pa. What will be its volume at 0°C and 101325Pa? (2marks)
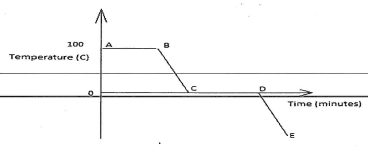
- The graph below is a cooling curve for water. Study it and answer the questions that follow.
- Explain what happens to the molecules of water in the region BC in terms of Kinetic theory. (2 marks)
- In what state is the water in the region DE? (1 mark)

Marking Scheme
-
- Saponification
- Vulcanization
- Nuclear fission
-
- Family of organic compounds whose members have similar structures, physical properties and chemical formula.
-
-
-
- Amount of solute that can form a saturated solution in 100g of water at a given temperature.
- 70g - 10g = 60g
If 80g of water ->60g of W
100g of water ->
=100 x 60
80
=75g/100g of water
-
- The amount of heat change involved when one mole of a solution completely dissolve in a solvent/water
-
-
-
- B and D
The drops/amount of soap needed to form lather remains the same even after boiling. - A and C
The drops/amount of soap needed to form lather decreased after boiling.
- B and D
- To some amount of dilute HNO5 in a beaker, add PbCO3 as you stir till in excess filter to obtain Pb(NO3)2 as filtrate.
Add NaCl solution to Pb(NO3)2 solution filter to obtain PbCl2 as residue.
Wash the residue with distilled water and dry between filter paper. -
- Fermentation
- Acidified Potassium manganate (VII) / H+ / KMnO4
Acidified potassium dichromate (VI) / H+ / K2Cr2O7 - Ethanol is reacted with concentrated H2SO4 acid at a temperature of 180oC for ethene and water to be formed.
- Sodium hydroxide conducts electric current by using mobile ions while graphite does by using delocalized electrons.
-
- To prevent the suck back.
To increase the surface over which the gas dissolves in water. - A white precipitate is formed.
Pb2+ ions react with Cl- ions to form the insoluble PbCl2
- To prevent the suck back.
-
- No effect;
The number of molecultes on both sides of the reaction are the same / total volumes of reactants and products are equal. - Equilibrium shifts to the right. Forward reaction is formed as an endothermic process will proceed.
- No effect;
-
-
- EMF = Ered - Eoxid
=+0.80 - (-2.37)
= +3.17v
-
-
- Zinc carbonate // ZnCO3
- ZnCO3(s) + 2HNO3(aq) → Zn(NO3)2(aq) + H2O(l) + CO2(g)
- Sodium Zincate
Na2ZnO2
-
-
- K
It is a strong alkali which dissociates completely in water - J
It is a weak acid
- K
-
-
-
-
- Water has strong hydrogen bonds between its molecules unlike carbon(IV)oxide which has weak Van der waal forces.
-
-
-
-
-
- RMM of Monomer = 42
n = 2000/42
=47.6
-
-
- Iron(III)oxide/FeO3/Silicon(IV)oxide/SiO2/Silica
- It reacts with acidic SiO2 and amphoteric Al2O3 so that Fe2O3 is removed as red mud
Reacts with impurites to form slag. - 2Al(OH)3(s) → Al2O3(s) + 3H2O(l)
-
-
- Copper (II) nitrate / Ca(NO3)2
- Ca(NO3)2(aq) + Na2CO3(aq) → CaCO3(s) + 2NaNO3(aq)
- A blue ppt is formed which dissolves to form a deep blue solution.
-
-
-
- Metals
They have smaller ionic radii than atomic radii - R
Has the largest atomic radius hence loses electrons most easily.
- Metals
-
- Universal indicator or litmus paper
-
Acid Base Neutral Lemon Juice Wood ash Sodium Chloride
-
- Amphoteric
- Aluminium, lead and zinc
-
- Rate increases
- Temperature increase increases kinetic energy of reacting particles hence increasing the number of fruitful collisions.
-
- Volume of a fixed mass of a gas is inversely proportional to its pressure provided the temperature remains the same.
-
-
- Molecules of water are losing heat/(the kinetic energy decreases) and molecules move closer to each other.
- Solid
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 1 Questions and Answers - Maranda Post Mocks 2020/2021.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students