Instructions to candidates
- Answer all the questions
- Non-programmable silent electronic calculators and KNEC mathematical tables may be used.
- All working must be clearly shown where necessary.
- Candidates should answer the questions in English.
- Crude oil is a mixture of hydrocarbons which are separated by fractional distillation. One of the components obtained contains an alkane A, with eleven carbon atoms.
- Write the molecular formula of A (1 mark)
- Pentane can be obtained from compound A as shown
A → Pentane + B- Give the name of this conversion process. (1 mark)
- State the conditions used in this process. (1 mark)
- Give the name of compound B. (1 mark)
- Draw and name two isomers of pentane (4 marks)
Isomer 1 Structure Name
Isomer 2 Structure Name - Incomplete combustion of pentane may result in air pollution. Write an equation to illustrate this combustion. (1 mark)
- The main component in natural gas is methane. Describe how methane in natural gas is formed. (2 marks)
- In the laboratory, methane can be prepared from salts of alkanoic acids. Describe how methane is prepared from sodium ethanoate. (2 marks)
-
-
- State what is meant by the term 'dynamic equilibrium. (1 mark)
- Dichromate(VI) ions are orange in colour while chromate(VI) ions are yellow. (i1) Consider the following equilibrium.
Cr2O72−(aq) + 2OH−(aq)2CrO42−(aq) + H2O(l)
State and explain the observation that will be made if sulphuric(VI) acid is added to the mixture. (2 marks)
- One of the reactions in the manufacture of nitric(V) acid involves catalytic oxidation of ammonia as shown in the equation.
4NH3(g)+ 5O2(g)4NO(g) + 6H2O(g); ΔH=−909 kJmol−1
The reaction is carried out at a pressure of 10 atmospheres and a temperature of 900°C- Other than nitric(V) acid, name another product that is formed. (1 mark)
- State and explain the effect on the position of equilibrium if the reaction is carried out.
- at 10 atmospheres pressure and 450°C; (2 marks) .
- at 900 °C and 20 atmospheres pressure; (2 marks)
- in the absence of a catalyst. (1 mark)
- State and explain the effect on the rate of the reaction if the reaction is carried out at 10 atmospheres and 450°C. (2 marks)
- A factory uses 100 kg of ammonia each day to produce 160 kg of nitrogen(II) oxide. Calculate the percentage yield of nitrogen(II) oxide. (3 marks)
-
-
- One of the ores of iron is haematite, Fe2O3. Give the name and formula of two other ores of iron. (2 marks)
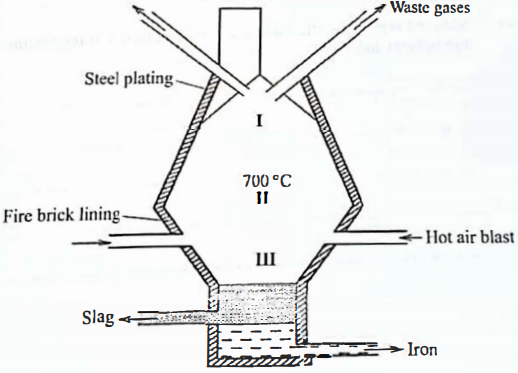
Name Formula - In a certain factory, iron is extracted from the haematite ore using the blast furnace as shown in Figure 1. The other raw materials are coke, limestone and air. The melting and boiling points of iron are 1535°C and 3000 °C, respectively.
Figure 1- State how the temperature in region I compares with that in region II. Give a reason. (1 mark)
- The main reducing agent in the furnace is carbon(II) oxide formed by the reaction
CO2(g) + C(s) →2C0g)
Write two equations to show how carbon(IV) oxide is formed in the furnace. (2 marks) - Suggest a value for the temperature in region III. Give a reason. (2 marks)
- Name the main component in the slag. (1 mark)
- State one role that slag plays in the blast furnace. (1 mark)
- The iron produced in the blast furnace is brittle due to presence of impurities
- Name the main impurity in this iron (1 mark)
- State one use of this iron (1 mark)
- Recycling is one method used to reduce production costs. State and explain the by products that can be recycled in this factory. (2 marks)
- One of the ores of iron is haematite, Fe2O3. Give the name and formula of two other ores of iron. (2 marks)
- Table 1 shows the elements in period 3 of the periodic table. Study it and answer the questions that follow.
Table 1Element Na Mg Al Si P S Cl Ar - Write the formulae of two oxides, for each of the following
- sodium: Oxide I... Oxide II... (1 mark)
- chlorine Oxide I ... Oxide II....(1 mark)
- The products of the reaction between phosphorus and chlorine depend on the conditions used. Write the equation for the reaction when chiorine reacts with excess phosphorus. (1 mark)
- Identify the element with the highest electrical conductivity. Give a reason. (2 marks)
- Describe an experiment that can be used to illustrate the variations in reaction of sodium, magnesium and aluminium with water. (3 marks)
- State and explain the differences in the melting points of:
- Chlorine and argon. (2 marks)
- magnesium oxide and silicon(IV) oxide. (2 marks)
- Write the formulae of two oxides, for each of the following
- Table 2 gives standard reduction potentials for some half cells.
Table 2
Half cell Half cell equation E0/V I Fe3+(aq) + e → Fe2+(aq) +0.77 II K+(aq) +e → K(s) −2.92 III Ag+(aq) + e → Ag(s) +0.80 IV Pb2+(aq) +2e → Pb(s) −0.13 V l2(aq) + 2e → 2l−(aq) +0.54 - State the standard conditions of an electrochemical cell. (2 marks)
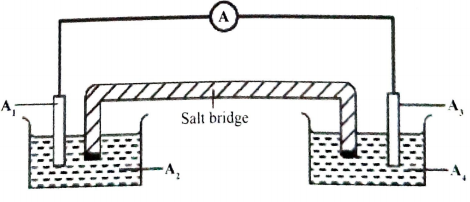
- An electrochemical cell was constructed using half-cells III and IV.
- Complete Figure 2 by labelling the parts of the cells indicated as A1- A4 (2 marks)
Figure 2
A1
A2
A3
A4 - Write an equation for the cell reaction and calculate the e.m.f. of the cell.
Equation (1 mark)
e.m.f. (1 mark)) - The salt bridge helps in completing the circuit. Explain why a saturated solution of potassium chloride is not suitable for use in the salt bridge in this electrochemical cell. (1 mark)
- Complete Figure 2 by labelling the parts of the cells indicated as A1- A4 (2 marks)
- State why it is not possible to construct a similar electrochemical cell using half-cells II and III. (1 mark)
- State and explain the observations made when aqueous potassium iodide is added to aqueous iron(III) sulphate. (2 marks)
- Acidified potassium dichromate(VI) and acidified potassium manganate(VII) may be used in determining concentration of Fe2+ ions in a sample. If acidified potassium dichromate(VI) is used, an indicator is added to determine the end point but for acidified potassium manganate(VII), no indicator is added.
- Explain why it is not necessary to use an indicator when acidified potassium manganate(VII) is used. (1 mark)
- An alloy containing iron was dissolved in an acid and the total volume made up to 250cm3. 25.0 cm3 of this solution required 18.0 cm3 of 0.15 M acidified potassium dichromate(VI) to react completely. The equation for the reaction is:
Cr2O72− (aq) + 6Fe2+(aq)+ 14H+(aq) →2Cr3+(aq)+ 6Fe3+(aq) +7H2O(l)
Calculate the mass of iron in the alloy (Fe = 56.0). (3 marks)
-
- water containing hydrogen carbonate, HCO3− and calcium Ca2+ ions, is said to be hard water
- Describe one way in which HCO3− ions get into river water (1 mark)
- Explain the disadvantage of using this type of water in boilers. (2 marks)
- Analysis of a river water sample showed the presence of the following ions Ca2+, Na+, Cl− , NO3−.
- Name the type of water hardness present in the sample. (1 mark)
- Describe one precipitation method that can be used to soften the water. (2 marks)
- The water sample was passed through a resin as shown in Figure 3.
- Write an equation for a reaction that took place in the column. (1 mark
- Complete treatment of the water sample required passing it through another resin. Give the formula of this resin. (1 mark)
- Explain why a river water sample that has been treated using resins may still require boiling to make it safe for drinking. (2 marks)
- Compound C was used to prepare a potassium soap.
- Give the formula of the potassium soap obtained. (1 mark)
- State one difference in the properties of potassium and sodium soaps. (1 mark)
- A soapless detergent has the formula
CH3(CH2)10CH2OSO3Na
With reference to this formula, identify the hydrophobic and the hydrophilic parts of the detergent. (1 mark)
Hydrophobic (1 mark)
Hydrophilic (1 mark)
- water containing hydrogen carbonate, HCO3− and calcium Ca2+ ions, is said to be hard water

Marking scheme
- Crude oil is a mixture of hydrocarbons which are separated by fractional distillation. One of the components obtained contains an alkane A, with eleven carbon atoms.
- Write the molecular formula of A (1 mark)
- C11 H24 ✓1
Accept open structural formula.
Acc. CH3(CH2)9CH2
- C11 H24 ✓1
- Pentane can be obtained from compound A as shown
A → Pentane + B- Give the name of this conversion process. (1 mark)
- Cracking ✓1
Acc. Catalytic/ Thermal cracking
- Cracking ✓1
- State the conditions used in this process. (1 mark)
- High temperatures ✓½/Acc. 400-700°C/Heat
- High pressure✓½ Acc. 70 atmospheres /Rej. Pressure alone.
- Catalyst✓½ Acc. Zeolite catalyst/Silica catalyst/Alumina catalyst
- Give the name of compound B. (1 mark)
- Hexene ✓1 Acc. C6 H12
Acc. Hex-1-ene/Hex-2-ene/Hex-3-ene.
- Hexene ✓1 Acc. C6 H12
- Give the name of this conversion process. (1 mark)
- Draw and name two isomers of pentane (4 marks)
Isomer 1 Structure Name
CH3CH2CH2CH2CH3 Pentane ✓1
CH3(CH2)3CH3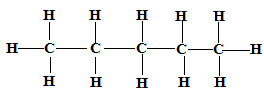 ✓1
✓1
Rej. molecular formula C5H12
(Name correct, wrong structure =0)
Isomer 2 Structure Name
2-methylbutane✓1
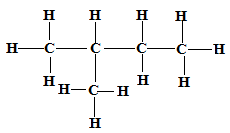 ✓1
✓1
2,2-dimethylpropane ✓1
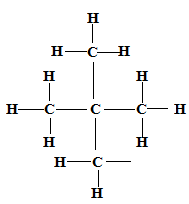 ✓1
✓1
Acc. condensed/open structure - Incomplete combustion of pentane may result in air pollution. Write an equation to illustrate this combustion. (1 mark)
- C5H12(g) + 3O2(g) → 5C(s) + 6H2O(l) ✓1
OR - 2C5H12(g) + 11O2(g) → 10CO(g) + 12H2O(l)
(Either one ingnore states
- C5H12(g) + 3O2(g) → 5C(s) + 6H2O(l) ✓1
- The main component in natural gas is methane. Describe how methane in natural gas is formed. (2 marks)
- Decomposition/breakdown/decay of organic matter ✓1 in the absence of oxygen ✓1
- In the laboratory, methane can be prepared from salts of alkanoic acids. Describe how methane is prepared from sodium ethanoate. (2 marks)
- Heat✓½ a mixture of sodium ethanoate and sodalime✓1
- Collect the gas over water ✓½ /use syringe/upward delivery/downward displacement of air.
(condition of heat must)
Accept correct diagram - Mixture of sodalime and ethanoate ✓1
- Arrow for heat ✓½
- Method of collection✓½
Accept equation CaO
Sodium ethanoate + sodalimeMethane + Na2CO ✓2
Heat
then mention method of collection
- Write the molecular formula of A (1 mark)
-
-
- State what is meant by the term 'dynamic equilibrium. (1 mark)
- State of reaction where the rate ✓1 of forward and backward reaction are the same but in opposite direction.
- Dichromate(VI) ions are orange in colour while chromate(VI) ions are yellow. (i1) Consider the following equilibrium.
Cr2O72−(aq) + 2OH−(aq)2CrO42−(aq) + H2O(l)
State and explain the observation that will be made if sulphuric(VI) acid is added to the mixture. (2 marks)- Intensity of orange colour increases
- Addition of H+ removes OH− hence reverse/backward reaction is favoured✓1 / equilibrium shifts to the left.
Accept concentration of OH− reduces.
- State what is meant by the term 'dynamic equilibrium. (1 mark)
- One of the reactions in the manufacture of nitric(V) acid involves catalytic oxidation of ammonia as shown in the equation.
4NH3(g)+ 5O2(g)4NO(g) + 6H2O(g); ΔH=−909 kJmol−1
The reaction is carried out at a pressure of 10 atmospheres and a temperature of 900°C- Other than nitric(V) acid, name another product that is formed. (1 mark)
- Nitrons acid/HNO2 ✓1
Nitric (III) Acid
- Nitrons acid/HNO2 ✓1
- State and explain the effect on the position of equilibrium if the reaction is carried out.
- at 10 atmospheres pressure and 450°C; (2 marks)
- Equilibrium shifts to the right✓1, forward reaction is favoured since it is exothermic✓1
- at 900 °C and 20 atmospheres pressure; (2 marks)
- Equilibrium shifts to the left✓1
- Backward reaction is favoured/favours ✓1 the direction with fewer molecules/due decrease in number of mole/molecules
- in the absence of a catalyst. (1 mark)
- No effect
- at 10 atmospheres pressure and 450°C; (2 marks)
- Other than nitric(V) acid, name another product that is formed. (1 mark)
- State and explain the effect on the rate of the reaction if the reaction is carried out at 10 atmospheres and 450°C. (2 marks)
- Rate of reaction decreases/reduces/slowers✓1.
- decrease in temperature leads to reduction/decrease in kinetic energy✓½ of the molecules hence less effective✓½ collisions.
- A factory uses 100 kg of ammonia each day to produce 160 kg of nitrogen(II) oxide. Calculate the percentage yield of nitrogen(II) oxide. (3 marks)
Moles of NH3 =100,000 ✓½ =5882.35
Moles of NO = mole ratio 1:1
Molar mass NH3 =17 ✓½ Molar mass N=30✓½
Mass of NO = 5882.35 × 30✓½ =176470.58
%yield of No = 160000 × 100 ✓½ OR 160 × 17 × 100
176470 30 × 100
=90.667% ✓½ =90.67%
-
-
- One of the ores of iron is haematite, Fe2O3. Give the name and formula of two other ores of iron. (2 marks)
Name Formula- Siderite ✓½ FeCO3✓½
- Magnetite✓½ Fe3O4✓½
Iron pyrite✓½ FeS2✓½
- In a certain factory, iron is extracted from the haematite ore using the blast furnace as shown in Figure 1. The other raw materials are coke, limestone and air. The melting and boiling points of iron are 1535°C and 3000 °C, respectively.
Figure 1- State how the temperature in region I compares with that in region II. Give a reason. (1 mark)
- Temp. in region I is lower than that in II ✓½
- Raw materials are not pre-heated ✓½
Region II is nearer to hot air blast that pre-heats
Hot air raises in the furnace it becomes cooler
- The main reducing agent in the furnace is carbon(II) oxide formed by the reaction
CO2(g) + C(s) →2C0g)
Write two equations to show how carbon(IV) oxide is formed in the furnace. (2 marks)- C(s) + O2(g) → CO2(g) ✓1
Heat - CaCO3(g)
CaO(s) +CO2(g) ✓1
(condition)
Fe2O2(s) + 3CO(g) → 2Fe(l) + 3CO2(g)
- C(s) + O2(g) → CO2(g) ✓1
- Suggest a value for the temperature in region III. Give a reason. (2 marks)
- 1535°C - 3000°C ✓1 Any value.
- Helps maintain Iron molten state. ✓1
- Name the main component in the slag. (1 mark)
- Calcium Silicate/ CaSiO3
- State one role that slag plays in the blast furnace. (1 mark)
- Prevents oxidation of iron by hot air .
- The iron produced in the blast furnace is brittle due to presence of impurities
- Name the main impurity in this iron (1 mark)
- Carbon
- State one use of this iron (1 mark)
- Making manhole covers
- Making scissors
- Bunsen burner bases
- Making iron pipes
- Electric poles
- Fire grills
- Manufacture of steel
- Manufacture of building blocks
- Electric fence arch
- Name the main impurity in this iron (1 mark)
- Recycling is one method used to reduce production costs. State and explain the by products that can be recycled in this factory. (2 marks)
- Waste gases✓1
- used to preheat the air blasts ✓1
- used to preheat the air blasts ✓1
- CO2(g)✓1
- used to preheat the air blasts. ✓1
- CO2 is converted to CO. Used as a reducing agent
- CO(g)✓1
- used as a reducing agent ✓1
- used to preheat air blasts.
- Waste gases✓1
- State how the temperature in region I compares with that in region II. Give a reason. (1 mark)
- One of the ores of iron is haematite, Fe2O3. Give the name and formula of two other ores of iron. (2 marks)
- Table 1 shows the elements in period 3 of the periodic table. Study it and answer the questions that follow.
Table 1Element Na Mg Al Si P S Cl Ar - Write the formulae of two oxides, for each of the following
- sodium: Oxide I Na2O Oxide II Na2O2 (1 mark)
- chlorine Oxide I Cl2O Oxide II Cl2O7 (1 mark)
ClO, ClO2, Cl2O2, Cl2O5, Cl2O6, Cl2O, ClO3
- The products of the reaction between phosphorus and chlorine depend on the conditions used. Write the equation for the reaction when chlorine reacts with excess phosphorus. (1 mark)
- 2P(s) + 3Cl2(g) → 2PCl3(l) ✓1
P4(s) + 6Cl2(g) → 4PCl3(g) ✓1
P10(s) + 15Cl2(g) → 10PCl3(g) ✓1
- 2P(s) + 3Cl2(g) → 2PCl3(l) ✓1
- Identify the element with the highest electrical conductivity. Give a reason. (2 marks)
- Al (Reject Al3+).
- Highest number of delocalised electrons/highest number of valence electrons per atom
- has 3 delocalised electrons per atom.
- Describe an experiment that can be used to illustrate the variations in reaction of sodium, magnesium and aluminium with water. (3 marks)
- Sodium reacts vigorously✓1 with water/hissing sound is produced
- Magnesium reacts slowly✓1with water/produces few bubbles on the surface.
- Aluminium does not react ✓1with water/produces no bubbles.
(Description is not a marking point)
- State and explain the differences in the melting points of:
- Chlorine and argon. (2 marks)
Melting point of chlorine is greater/higher ✓1than that of argon. Cl2(g) is diatomic✓½ while Ar(g) is monoatomic hence Cl2(g) has stronger✓½ vanderwaals. Forces of attraction/molecules of Cl2(g) are larger/bigger than Ar(g) molecules hence intermolecules forces in Cl2 are stronger than Ar. - magnesium oxide and silicon(IV) oxide. (2 marks)
- Magnesium oxide has a higher melting than Silicon (IV) Oxide.✓2
- Chlorine and argon. (2 marks)
- Write the formulae of two oxides, for each of the following
- Table 2 gives standard reduction potentials for some half cells.
Table 2
Half cell Half cell equation E0/V I Fe3+(aq) + e → Fe2+(aq) +0.77 II K+(aq) +e → K(s) −2.92 III Ag+(aq) + e → Ag(s) +0.80 IV Pb2+(aq) +2e → Pb(s) −0.13 V l2(aq) + 2e → 2l−(aq) +0.54 - State the standard conditions of an electrochemical cell. (2 marks)
- 1M solution
- 1 atmospheric pressure.
- Temperature of 25°C or 278K
3 correct 2mks
2 correct 1 mk
1 correct ½ mk
- An electrochemical cell was constructed using half-cells III and IV.
- Complete Figure 2 by labelling the parts of the cells indicated as A1- A4 (2 marks)
Figure 2
Soluble salt of Pb
A1 Pb/Lead electrode ✓½
A2 Pb2+/ Lead(II)Nitrate solution/1M Pb2+ ✓½
Soluble salt of Ag
A3 Ag/ Silver electrode ✓½
A4 Ag+/ Silver Nitrate/1M Ag+ ✓½ - Write an equation for the cell reaction and calculate the e.m.f. of the cell.
- Equation (1 mark)
Pb(s) + 2Ag+ (aq) → Pb2+ (aq) + 2Ag(s) ✓1 - e.m.f. (1 mark)
+0.8−−0.13 ✓½
+0.93V✓½
- Equation (1 mark)
- The salt bridge helps in completing the circuit. Explain why a saturated solution of potassium chloride is not suitable for use in the salt bridge in this electrochemical cell. (1 mark)
- Formation of insoluble PbCl2✓½ That reduces the concentration of ions✓½ in electrolyte
OR - Formation of AgCl that reduces the effectiveness of the cell
- Formation of insoluble PbCl2✓½ That reduces the concentration of ions✓½ in electrolyte
- Complete Figure 2 by labelling the parts of the cells indicated as A1- A4 (2 marks)
- State why it is not possible to construct a similar electrochemical cell using half-cells II and III. (1 mark)
- K reacts explosively with water.✓1
Acc. E.M.F of cell is very high which can explode with the cell.
- K reacts explosively with water.✓1
- State and explain the observations made when aqueous potassium iodide is added to aqueous iron(III) sulphate. (2 marks)
- (Yellow) Brown solutions✓½ turns to green. Fe3+ ions✓½ are reduced to Fe2+
- Grey/black precipitate✓½ is formed. Iodide ions✓½ are oxidised to iodine
Acc.Equation - Fe3+(aq) + 2I−(aq) → Fe2+(aq) + I2(s) is +0.23V
Brown/yellow✓½solubles Green✓½ Grey/black✓½ Explanation✓½
- Acidified potassium dichromate(VI) and acidified potassium manganate(VII) may be used in determining concentration of Fe2+ ions in a sample. If acidified potassium dichromate(VI) is used, an indicator is added to determine the end point but for acidified potassium manganate(VII), no indicator is added.
- Explain why it is not necessary to use an indicator when acidified potassium manganate(VII) is used. (1 mark)
- H+/KMnO4, acts as its own indicator changing from purple to colourless✓1 (decolourised)
- An alloy containing iron was dissolved in an acid and the total volume made up to 250cm3. 25.0 cm3 of this solution required 18.0 cm3 of 0.15 M acidified potassium dichromate(VI) to react completely. The equation for the reaction is:
Cr2O72− (aq) + 6Fe2+(aq)+ 14H+(aq) →2Cr3+(aq)+ 6Fe3+(aq) +7H2O(l)
Calculate the mass of iron in the alloy (Fe = 56.0). (3 marks)
Moles of Cr2O72−=18 × 0.15 ✓½
1000 =0.0027
Moles of Fe2+ in 25.0cm3 = 6 × 0.0027 = 0.0162✓½
Moles of Fe2+ in 250cm3 = 0.0162 × 10✓½ = 0.162✓½
0.648M in 250cm3
M=0.0162 × 1000
25
=0.648M
Mass of Iron=0.162 × 56✓½
=9.072g✓½
Condensed working
18 × 0.15 × 6 × 250 × 56 = 9.072g
1000×25
(All marks awarded)
- Explain why it is not necessary to use an indicator when acidified potassium manganate(VII) is used. (1 mark)
- State the standard conditions of an electrochemical cell. (2 marks)
-
- water containing hydrogen carbonate, HCO3− and calcium Ca2+ ions, is said to be hard water
- Describe one way in which HCO3− ions get into river water (1 mark)
- CO2 dissolves in rain water to form Carbonic acid ✓½
- Carbonic acid reacts with carbonate rock/Ca and Mg forming hydrogen carbonates of Ca and Mg that gets into rivers. ✓½
- Explain the disadvantage of using this type of water in boilers. (2 marks)
- The CaHCO3 and MgCO3 decomposes forming CaCO3/scales/fur ✓1 lining the boilers and causing poor thermal conductivity/reducing efficiency.
- Describe one way in which HCO3− ions get into river water (1 mark)
- Analysis of a river water sample showed the presence of the following ions Ca2+, Na+, Cl− , NO3−.
- Name the type of water hardness present in the sample. (1 mark)
- Permanent
- Describe one precipitation method that can be used to soften the water. (2 marks)
- Addition of washing soda/NaCO3✓1
- Ca2+and Mg2+ precipitates as carbonates which can be filtered off✓1
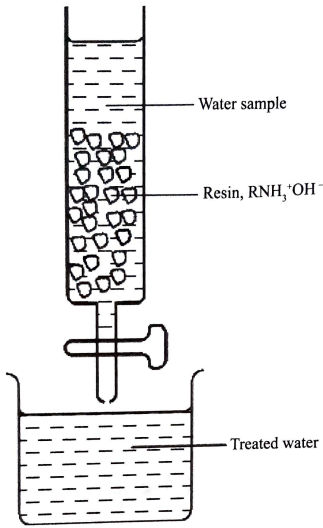
- The water sample was passed through a resin as shown in Figure 3.
- Write an equation for a reaction that took place in the column. (1 mark)
Resin
RNH3+OH−(s) + NO3−(aq) or Cl−→ RNH3+NO−(s)(Cl−if its over used) + OH(aq)
(show changes and balance/ Ignore states) - Complete treatment of the water sample required passing it through another resin. Give the formula of this resin. (1 mark)
- RSO3−H+ OR RCOO−H+ (Ignore changes)
- Explain why a river water sample that has been treated using resins may still require boiling to make it safe for drinking. (2 marks)
- Resin does not kill bacteria/pathogen✓1
- Boiling kills✓1
- Write an equation for a reaction that took place in the column. (1 mark)
- Name the type of water hardness present in the sample. (1 mark)
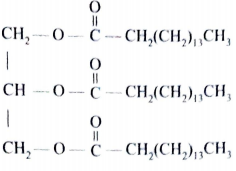
- Compound C was used to prepare a potassium soap.
- Give the formula of the potassium soap obtained. (1 mark)
- C15H31COOK/ CH3(CH2)14 COOK/ CH3(CH2)15 CH2 COOK
- State one difference in the properties of potassium and sodium soaps. (1 mark)
- Potassium soaps lather more readily in water than Na soaps.
- Potassium soaps have lower/less melting points.
- Potassium salts are more soluble in water.
- Potassium soaps are soft/mild while sodium soaps are hard.
- Give the formula of the potassium soap obtained. (1 mark)
- A soapless detergent has the formula
CH3(CH2)10CH2OSO3Na
With reference to this formula, identify the hydrophobic and the hydrophilic parts of the detergent. (1 mark)- Hydrophobic (1 mark)
- CH3(CH2)10CH2− OR (CH3)3(CH2)11−
Reject CH3(CH3)10
- CH3(CH2)10CH2− OR (CH3)3(CH2)11−
- Hydrophilic (1 mark)
- OSO3−Na+ OR OSO3Na
Reject CH2OSO3−Na+
- OSO3−Na+ OR OSO3Na
- Hydrophobic (1 mark)
- water containing hydrogen carbonate, HCO3− and calcium Ca2+ ions, is said to be hard water
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 2 Questions and Answers - KCSE 2020 past papers.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students