SUKELLEMO JOINT MOCK
Kenya Certificate of Secondary Education
CHEMISTRY PAPER 2
(THEORY)
2 hours
Instructions to Candidates
- Answer all the questions in the spaces provided.
- All working must be clearly shown.
- Non-programmable silent electronic calculators and KNEC mathematical tables may be used.
Answer all the questions in the spaces provided
-
- Study the standard electrode potential for the half-cells given below and answer the questions that follow. (The letters do not represent the actual symbols of the elements)
Half-Cells Eº(Volts) N+(aq) + e- → N(s) -2.92 J+(aq) + e- → J(s) +0.52 K+(aq) + e- → K(s) 0.00 1/2 G2(g) + e- → G-(s) +1.36 M2+(aq) + 2e- → M(s) -0.44 - Identify the strongest reducing agent. Give a reason for your answer.( 2 marks)
- Which two half- cells would produce the highest potential difference when combined? (1 mark)
- Explain whetether the reaction represented below can take place. ( 2 marks)
2N+(aq) + M(s) → 2N (s) + M2+(aq)
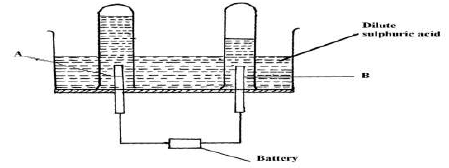
- 100cm3 of 2M sulphuric (VI) acid was electrolyzed using the set up represented by the diagram below.
- Name electrode A and electrode B (2 marks)
A
B - Write an equation for the reaction that produces gas L. (1 mark)
- Describe how gas K can be identified (1 mark)
- Explain the difference in:
- The volume of the gases produced at the electrodes. (1 mark)
- Brightness of the bulb if 100 cm3 of 2M ethanoic acid was used in place of sulphuric (VI) acid. (2 marks)
- Name electrode A and electrode B (2 marks)
- Study the standard electrode potential for the half-cells given below and answer the questions that follow. (The letters do not represent the actual symbols of the elements)
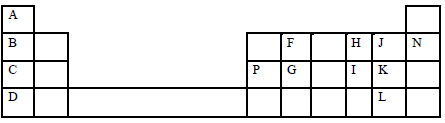
- The grid below presents part of the periodic table. Study it and answer the questions that follow. The letters are not actual symbols of the elements.
- State the family name of the following elements B, K and N. (3 marks)
B-
K-
N- - Give the formula of the compound formed between P and K. (1 mark)
- Compare and explain the melting points of elements C, P and G. (2 marks)
- Name the most reactive metallic and non- metallic elements. (1 mark)
Metallic-
Non-metallic – - Write the equation for the reaction that takes place between element C and water.(1 mark)
- Compare and explain the first ionization energies of elements C and D. ( 2 marks)
- Element B combines with chlorine to form a chloride of B. State the most likely pH value of a solution of a chloride of B. Explain ( 2 marks)
- State the family name of the following elements B, K and N. (3 marks)
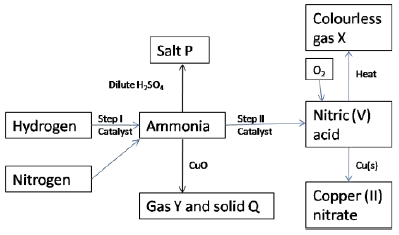
- Study the scheme below and answer the questions that follow.
- State one source of each of the following (2 marks)
- Hydrogen
- Nitrogen
- State two other conditions other than the use of catalyst that would favour the reaction in step I ( 2 marks)
- Name the catalyst used in each of the steps I and II (2 marks)
Step I
Step II - Name the following substances
- Salt P (1 mark)
- Gas X (1 mark)
- Solid Q(1 mark)
- Gas Y(1 mark)
- State one source of each of the following (2 marks)
-
- The following data was obtained during an experiment to determine the molar heat of combustion of ethanol.
Volume of water used =500cm3
Initial temperature of water =25oc
Final temperature of water = 44.5oc
Mass of ethanol + lamp before burning = 121.5g
Mass of ethanol+ lamp after burning = 120.0g
Calculate the;- The highest temperature change. (1 mark)
- The mass of ethanol used to boil water. (1 mark)
- Number of moles of ethanol used. (molar mass of ethanol=46.0g) (1 mark)
- Heat evolved during the experiment (density of water-1g/cm3, specific heat capacity of water=4.2Jg-1K-1). (2 marks)
- Molar heat of combustion of ethanol (C=12,O=16, H=1) (2 marks)
- Write the thermochemical equation for the complete combustion of ethanol. (1 mark)
- In the spaces provided, sketch a simple energy level diagram for the above change. (2 marks)
- The following data was obtained during an experiment to determine the molar heat of combustion of ethanol.
- The table below contains information from the measurements made of the radioactivity in counts per minute from radioisotope iodine-128.
Counts per minute 240 186 170 156 143 122 108 Time (minutes) 0 15 20 25 30 40 50 - Plot a graph of counts per minutes against time. (3 marks)
- Use the graph to determine the half-life of iodine 128. (1 mark)
- What is the count rate after: (2 marks)
- 12 minutes?
- 22 minutes?
- After how many minutes was the count rate: ( 2 marks)
- 160 counts per minute?
- 197 counts per minute?
- State two uses of radioactive isotopes in agriculture. (2marks)
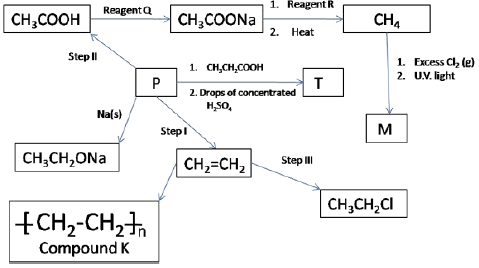
- The scheme below shows some reactions starting with ethane. Study it and answer the following questions.
- Give the name and draw the structural formula of compound P. (2 marks)
- Name the type of reaction and the reagents for the reactions in the following steps.
- Step I Type (2 marks)
Reagents - Step II Type (2 marks)
Reagents - Step III Type (2 marks)
Reagents
- Step I Type (2 marks)
- Name the reagent Q(1 mark)
- Give the name and the structure of compound T (2 marks)
- Structure
- Name
- Draw the structural formula of M and give name (1 mark)
- Structure
- Name
-
- Name compound K (1 mark)
- If the relative molecular mass of K is 84,000 determine the value of n (C=12, H=1) (1 mark)
-
- Name the allotropes of carbon. (1 mark)
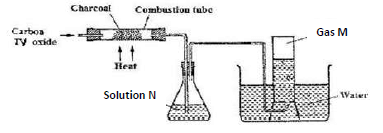
- Carbon (IV) oxide was passed over heated charcoal powder as shown in the set up below
- Name gas M (1 mark)
- Write an equation for the formation of gas M (1 mark)
- Identify solution N and state its purpose in the set up. (2 marks)
- Carbon (IV) oxide does not support combustion yet burning magnesium continues to burn in it.
- Explain this observation (1 mark)
- Write a chemical equation for the reaction that occurs. (1 mark)
- Using dots (.) and cross (x) to represent outermost electrons, show the structure of a carbon (IV) oxide molecule. (2 marks)
- Carbon (IV) oxide is used in the industrial manufacture of sodium carbonate.
- Name the other reagent in the Solvay process. (1 mark)
- Name the by product in this process and state any two of its uses. ( 2 marks)
Download Chemistry Paper 2 Questions and Answers - Sukellemo Joint Mock 2020/2021.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students