- Answer all the questions in the spaces provided
- Mathematical tables and calculators may be used
- ALL workings must be clearly shown where necessary
- Study the data in the table below and answer the questions. The letters do not represent the actual symbols of the elements.
Element Atomic number Boiling point °C P 3 1333 Q 13 2470 R 16 445 S 18 −186 T 19 774 - Select the elements that belong to the same
- Group (1 mk)
- Period (1 mk)
- Which element:
- Is a gas at room temperature? Explain. (2 mks)
- Does not take an oxide (1 mk)
- Write the:
- Formula of the Sulphate of element Q (1 mk)
- Equation for the reaction between elements P and R (1 mk)
- What type of bonding would exist in the compound formed when elements R and Q react? Give a reason for your answer. (2 mks)
- Select the most electropositive element (1mk)
- Explain why the boiling point of element Q is higher than that of element P. (2 mks)
- Select the elements that belong to the same
- In order to determine the imolar heat of neutralization of potassium hydroxide, 200cm3 of 1M Hydrochloric acid and 200cm3 Of IM Potassium hydroxide both at the same initial temperature were mixed and stirred continuously with a thermometer. The temperature of the resulting solution was recorded after every 30 seconds until the highest temperature of the solution was attained. Thereafter, the temperature of the solution was recorded for a further two minutes.
-
- Why was it necessary to stir the mixture of the two solutions? (1 mk)
- Define molar heat of neutralization (1 mk)
- Write an ionic equation for the reaction which took place (1 mk)
- If the initial temperature for both solution was 24.5°C and the highest temperature attained by the mixture was 30.9°C.
Calculate the:- Heat change for the reaction (specific heat capacity of the solution
= 4.2Jg−1K−1and the density of the solution = 1.0g/cm3 (2 mks) - Molar heat at neutralization of potassium hydroxide. (2 mks)
- Heat change for the reaction (specific heat capacity of the solution
- Explain how the value of the molar heat of neutralization obtained in the experiment would compare with the one that would be obtained if the experiment was repeated using 200cm3 of 1 Mammonium hydroxide instead of IM potassium hydroxide. (2 mks)
- Use the following information to answer the questions that follow:
C(s) + O2(g) → CO2(g) ∆H = -393KJ/mol
H2(g) + ½O2(g) → H20(g) ∆H = -296KJ/mol
13
C4H10+ 13/2O2(g) → 4CO2(g)+ 5H20 ∆H =-2877K]moi
Calculate the heat of formation of butane. (3 mrks)
-
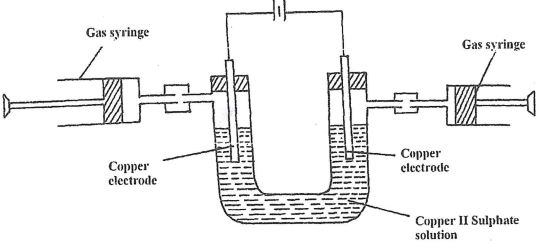
- Aqueous Copper (II) Sulphate was electrolyzed using the set up represented by the diagram below
- After sometimes it was found that no gas was produced at both electrodes. Explain. (1 mk)
- Write an equation for the reaction at each electrode. (2 mks)
- Anode
- Cathode
- What happens to the colour of the electrolyte during electrolysis. Explain (2 mks)
- An iron spoon is to be electroplated with silver. Draw a labeled diagram of the set-up that could be used to represent the process. (2 mks)
- The following are half-cell equations for some elements. The letters do not represent actual symbols. Use the information to answer the questions that follow.
Half cell Eθ, v
M2+(aq)+ 2ē → M(s) +0.34
L2+(aq)+2ē → L(s) +0.84
K2+(aq)+ 2ē → K(s) -0.13
J2+(aq) + 2ē + J(s) -0.76- Select the two half cells that would produce the highest e.m.f of a cell. (1 mk)
- Calculate the e.m.f of the cell in d(i) above. (2 mks)
- Give the cell diagram notation for the cell in d(ii) above. (1 mk)
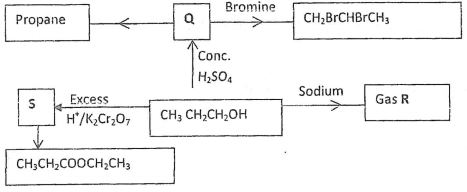
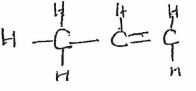
- The scheme below shows several reactions starting with propanol. Study the scheme and answer the questions which follow.
- Name gas R (1 mk)
- Name and draw the structural formula of compound Q. (2 mk)
- What conditions and reagents are necessary to convert S to CH3CH2COOCH2CH3 (2 mks)
Reagents Conditions - Write an equation for the reaction that takes place when equal volumes of chlorine gas react with propane. (1mk)
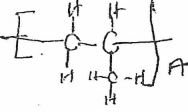
- The table below shows some properties of organic compounds U, V and W. Use the information to answer the questions that follow.
To which homologous series do the compounds belong? (3 mks)W V U Reaction with liquid bromine Decolourises bromine very fast No reaction Decolourises bromine liquid slowly Combustion Burns with yellow smoky flame Burns with a blue flame leaving no residue Burns with a yellow sooty flame Reaction with Conc.H2SO4 No reaction It is dehydrated to form compound U Reacts to form V
U-
V-
W- - CH2 = CH – CH3 when heated under high temperatures and pressures forms a solid with large molecular mass.
- Write the equation for the reaction which involves the formation of the solid. (1 mk)
- Name the solid and give one use of the solid (2 mks)
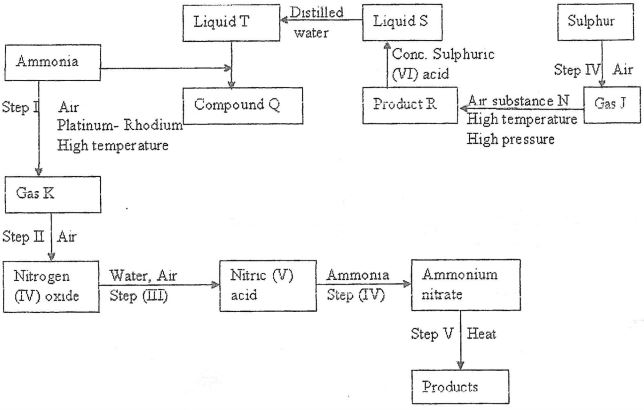
- Study the flow chart below and answer the questions that follow
- Identify gas:
- J..... (1mk)
- K... (1mk)
- Write the equation for the reaction that occurs in steps:
(V)........(1mk)
(VI)... (1mk) - Name substance N.....(1mk)
- Write an equation for formation of R and explain why high pressure is required in converting J to R (3 mks)
- Give one commercial use of;
- Liquid T........(1mk)
- Compound Q....(1mk)
- Describe a chemical test that would be used to distinguish the anion in compound Q and the product in Step IV (2 mks)
- State and explain the observation made when concentrated sulphuric (VI) is added to crystals of copper (II) Sulphate in a beaker. (2 mks)
- Identify gas:
-
-
- Give the name of the reagent which when reacted with concentrated hydrochloric acid produce chlorine gas. (1 mk)
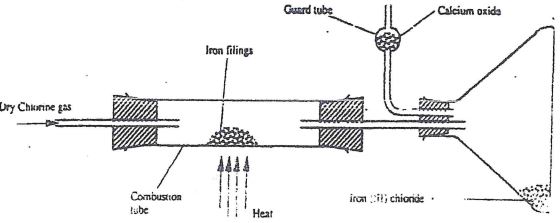
- A student out to prepare iron III chloride using the apparatus shown in the diagram below.
- Explain why:
- It is necessary to pass chlorine gas through the apparatus before heating begins. (1 mk)
- Calcium oxide would be preferred to calcium chloride in the guard tube. (1 mk)
- What property of iron (III) chloride makes it possible to be collected as shown in the diagram? (1mk)
- Write an equation form one chemical reaction that took place in the guard tube (1mk)
- The total mass of iron (III) chloride formed was found to be 0.5g.
Calculate the volume of chlorine gas the reacted with iron.
(Fe - = 56.0, C1 = 35.5 and Molar gas volume at 298K is 24,000cm3 (3 mk)
- Explain why:
-
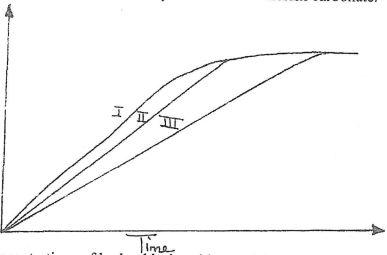
- Below is a graph that was obtained when different concentrations of hydrochloric acid was reacted with equal amount of calcium carbonate.
The concentrations of hydrochloric acid were 0.8m, 0.5m and 0.1m. The calcium carbonate was in powder form. Match the graphs with concentrations.
Graph I (1 mk)
Graph II (1 mk) - A state of equilibrium between dichromate (VI) and chromate ions is established as shown in the equation below.
Cr2O7 (aq) + 20H−(aq)2CrO4(aq)+ H2O(l)
Orange Yellow
State and explain observation made when a few pellets of potassium hydroxide are added to the equilibrium mixture. (2 mks)
- Below is a graph that was obtained when different concentrations of hydrochloric acid was reacted with equal amount of calcium carbonate.
-
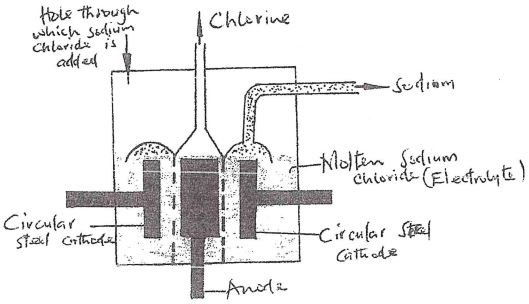
- The above is a simplified diagram of the Downs Cell used for the manufacture of sodium. Study it and answer the questions that follow.
- What material is the anode made of? Give a reason (2 mks)
- What precaution is taken to prevent chlorine and sodium from re- combination? (1 mk)
- Write an ionic equation for the reaction in which chlorine gas is formed (1 mk)
- In the Downs process, (used for manufacture of sodium), a certain salt is added to lower the melting point of sodium chloride from about 800°C to about 600°C.
- Name the salt that is added (1 mk)
- State why it is necessary to lower the temperature (1mk)
- Explain why aqueous sodium chloride is not suitable as an electrolyte for the manufacture of sodium in the Downs process (2 mks)
- Sodium metal reacts with air to form two oxides. Give the formulae of two oxides (1 mk)
- The above is a simplified diagram of the Downs Cell used for the manufacture of sodium. Study it and answer the questions that follow.

Marking Scheme
- Study the data in the table below and answer the questions. The letters do not represent the actual symbols of the elements.
Element Atomic number Boiling point °C P 3 2.1 1333 Q 13 2.8.3 2470 R 16 2.8.6 445 S 18 2.8.8 −186 T 19 2.8.8.1 774 - Select the elements that belong to the same
- Group (1 mk)
- P and T
- Period (1 mk)
- Q,R & S
- Group (1 mk)
- Which element:
- Is a gas at room temperature? Explain. (2 mks)
- S, It's boiling point is below room temperature
- Does not take an oxide (1 mk)
- S
- Is a gas at room temperature? Explain. (2 mks)
- Write the:
- Formula of the Sulphate of element Q (1 mk)
- Q2(SO4)3
- Equation for the reaction between elements P and R (1 mk)
- 2P(s)+R(s)→P2R(s)
- Formula of the Sulphate of element Q (1 mk)
- What type of bonding would exist in the compound formed when eiements R and Q react? Give a reason for your answer.
(2 mks)- Ionic
Q transfers it's electrons to R when they react.
- Ionic
- Select the most electropositive element (1mk)
- T
- Explain why the boiling point of element Q is higher than that of element P. (2 mks)
- Q has strong metallic bond than P, it has more delocalised electrons hence strong metallic bond
- Select the elements that belong to the same
- In order to determine the inolar heat of neutralization of potassium hydroxide, 200cm3 of 1M Hydrochloric acid and 200cm3 Of IM Potassium hydroxide both at the same initial temperature were mixed and stirred continuously with a thermometer. The temperature of the resulting solution was recorded after every 30 seconds until the highest temperature of the solution was attained. Thereafter, the temperature of the solution was recorded for a further two minutes.
-
- Why was it necessary to stir the mixture of the two solutions? (1 mk)
- To ensure heat is evenly distributed in the solution
- Define molar heat of neutralization (1 mk)
- Enthalpy change when 1mole of a OH−are neutralized by 1mole of H+ to form 1 mole of water
- Write an ionic equation for the reaction which took place (1 mk)
- OH−(aq)+ H+(aq)→H2O(l)
- Why was it necessary to stir the mixture of the two solutions? (1 mk)
- If the initial temperature for both solution was 24.5°C and the highest temperature attained by the mixture was 30.9°C.
Calculate the:- Heat change for the reaction (specific heat capacity of the solution
= 4.2Jg−1K−1and the density of the solution = 1.0g/cm3) (2 mks)
∆H =400 × 4.2 × 6.4
=10752J//10.75% - Molar heat at neutralization of potassium hydroxide. (2 mks)
Moles of KOH =200 × 1
1000
=0.2
0.2→10.752
1mole→?
10.752 × 1
0.2
=−53.76KJ/mole
- Heat change for the reaction (specific heat capacity of the solution
- Explain how the value of the molar heat of neutralization obtained in the experiment would compare with the one that would be obtained if the experiment was repeated using 200cm3 of 1 Mammonium hydroxide instead of IM potassium hydroxide.
(2 mks)- Ammonium hydroxide is a weak base, it's partially ionized some of the heat is used up to fully ionize. Heat of KOH is higher than ammonium hydroxide
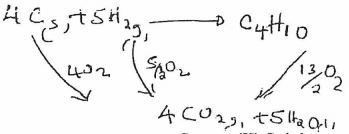
- Use the following information to answer the questions that follow:
C(s) + O2(g) → CO2(g) AH = -393KJ/mol
H2(g) + ½O2(g) → H20(g) AH = -296KJ/mol
C4H10+ 13/2O2(g) → 4CO2(g)+ 5H20 AH =-2877K]moi
Calculate the heat of formation of butane. (3 mrks)
∆H=4x − 393 + 5x −296−−2877
=−1572+−1480−−2877
=−175KJ/mole
-
- Aqueous Copper (II) Sulphate was electrolyzed using the set up represented by the diagram below
- After sometimes it was found that no gas was produced at both electrodes. Explain. (1 mk)
- At the cathode,Cu2+ are preferential discharged
- At the anode Cu solid is discharged
- Write an equation for the reaction at each electrode. (2 mks)
- Anode
- Cu(s) → Cu2+(aq) + 2e−
- Cathode
- Cu2+(aq) + 2e− → Cu(s)
- Anode
- What happens to the colour of the electrolyte during electrolysis. Explain (2 mks)
- Blue color remains blue Cu2+ removed are replaced by Cu dissolving at anode
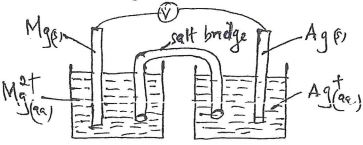
- An iron spoon is to be electroplated with silver. Draw a labeled diagram of the set-up that could be used to represent the process. (2 mks)
- The following are half-cell equations for some elements. The letters do not represent actual symbols. Use the information to answer the questions that follow.
Half cell Eθ, v
M2+(aq)+ 2ē → M(s) +0.34
L2+(aq)+2ē → L(s) +0.84
K2+(aq)+ 2ē → K(s) -0.13
J2+(aq) + 2ē + J(s) -0.76- Select the two half cells that would produce the highest e.m.f of a cell. (1 mk)
L(s)/L2+(aq), a-d J(s)/J2+(aq) - Calculate the emf of the cell in d(i) above. (2 mks)
+0.84−−a76 =+1.60v - Give the cell diagram notation for the cell in d(ii) above. (1 mk)
J(s)/J2+(aq)//L(s)/L2+(aq),Eθ=1.60v
- Select the two half cells that would produce the highest e.m.f of a cell. (1 mk)
- After sometimes it was found that no gas was produced at both electrodes. Explain. (1 mk)
- The scheme below shows several reactions starting with propanol. Study the scheme and answer the questions which follow.
- Name gas R (1 mk)
- Hydrogen
- Name and draw the structural formula of compound Q. (2 mks)
Propene - What conditions and reagents are necessary to convert S to CH3CH2COOCH2CH3 (2 mks)
Reagents Ethanol Conditions 1. Drops of Conc. H2SO4 2. Warm - Write an equation for the reaction that takes place when equal volumes of chlorine gas react with propane. (1 mk)
CH3CH2CH3(g) + Cl2(g) → CH3CH2CH2Cl +HCL - The table below shows some properties of organic compounds U, V and W. Use the information to answer the questions that follow.
To which homologous series do the compounds belong? (3 mks)W V U Reaction with liquid bromine Decolourise bromine very fast No reaction Decolourises bromine liquid slowly Combustion Burns with yellow smoky flame Burns with a blue flame leaving no residue Burns with a yellow sooty flame Reaction with Conc.H2SO4 No reaction It is dehydrated to form compound U Reacts to form V
U-Alkene
V-Alkanol
W-Alkyne - CH2 = CH – CH3 when heated under high temperatures and pressures forms a solid with large molecular mass.
- Write the equation for the reaction which involves the formation of the solid. (1 mk)
n(CH2=CHCH3)→ - Name the solid and give one use of the solid (2 mks)
- Polypropene
Making ropes,crates
- Polypropene
- Write the equation for the reaction which involves the formation of the solid. (1 mk)
- Name gas R (1 mk)
- Study the flow chart below and answer the questions that follow
- Identify gas:
- J Sulphur (IV) Oxide (1mk)
- K Nitrogen(II)Oxide (1mk)
- Write the equation for the reaction that occurs in steps:
(V) NH4NO3(s)→ N2O(g) + 2H2O(l) (1mk)
(VI) SO3(g) + H2SO4(l) → H2S2O7(l) (1mk) - Name substance N Vanadium (VI) Oxide (1mk)
- Write an equation for formation of R and explain why high pressure is required in converting J to R (3 mks)
2SO2(g)+O2(g) → 2SO3(g)
Forward reaction leads to reduction in volume, high pressure is required to reduce the volume - Give one commercial use of;
- Liquid T Manufacture of fertilizer, Dyes, drugs, paints (1mk)
- Compound Q As a fertilizer (1mk)
- Describe a chemical test that would be used to distinguish the anion in compound Q and the product in Step IV (2 mks)
Product SO42−/NO3−
Chemical test; To each sample add barium Nitrate for SO42− a white preicpitate is formed for NO3−,no white precipitate - State and explain the observation made when concentrated sulphuric (VI) is added to crystals of copper (II) Sulphate in a beaker. (2 mks)
- Blue crystals changes to white powder
- Conc H2SO4 dehydrates hydrated copper sulphate
- Identify gas:
-
-
- Give the name of the reagent which when reacted with concentrated hydrochloric acid produce chlorine gas. (1 mk)
- Lead(IV)Oxide/ Manganese(IV)Oxide/Potassium Manganate(IV)
- A student out to prepare iron III chloride using the apparatus shown in the diagram below.
- Explain why:
- It is necessary to pass chlorine gas through the apparatus before heating begins. (1 mk)
- To prevent iron from being oxidized
- Calcium oxide would be preferred to calcium chloride in the guard tube. (1 mk)
- It also absorbs Cl2, preventing it from escaping to the atmosphere.
- It is necessary to pass chlorine gas through the apparatus before heating begins. (1 mk)
- What property of iron (III) chloride makes it possible to be collected as shown in the diagram? (1 mk)
- It sublimes when heated
- Write an equation form one chemical reaction that took place in the guard tube (1mk)
Cl2(g) +CaO(s) → CaOCl2(s) - The total mass of iron (III) chloride formed was found to be 0.5g.
Calculate the volume of chiorine gas the reacted with iron. (3 mk)
(Fe - = 56.0, C1 = 35.5 and Molar gas volume at 298K is 24,000cm3
2Fe(s) + 3Cl2(g) → 2FeCl3(s)
Moles of FeCl3 = 0.5/162.5
=0.00308moles
Mole ratio
FeCl3: Cl2
2:3
0.00308 ?
0.00308×3/2
0.00462 × 24000
=110.88cm3
- Explain why:
- Give the name of the reagent which when reacted with concentrated hydrochloric acid produce chlorine gas. (1 mk)
-
- Below is a graph that was obtained when different concentrations of hydrochloric acid was reacted with equal amount of calcium carbonate.
The concentrations of hydrochloric acid were 0.8m, 0.5m and 0.1m. The calcium carbonate was in powder form. Match the graphs with concentrations.
Graph I 0.8 (1 mk)
Graph II 0.5 (1 mk) - A state of equilibrium between dichromate (VI) and chromate ions is established as shown in the equation below.
Cr2O7 (aq) + 20H−(aq)2CrO4(aq)+ H2O(l)
Orange Yellow
State and explain observation made when a few pellets of potassium hydroxide are added to the equilibrium mixture. (2 mks)- Mixture becomes yellow
- KOH increases concentration of OH− hence forward reaction is favoured
- Below is a graph that was obtained when different concentrations of hydrochloric acid was reacted with equal amount of calcium carbonate.
-
-
- The above is a simplified diagram of the Downs Cell used for the manufacture of sodium. Study it and answer the questions that follow.
- What material is the anode made of? Give a reason (2 mks)
- Graphite
Does not react with products of electrolysis.
- Graphite
- What precaution is taken to prevent chlorine and sodium from re- combination? (1 mk)
- Steel gauze diaphram is used to separate the Anode from cathode
- Write an ionic equation for the reaction in which chlorine gas is formed (1 mk)
2Cl(aq) → Cl2(g) + 2e−
- What material is the anode made of? Give a reason (2 mks)
- In the Downs process, (used for manufacture of sodium), a certain salt is added to lower the melting point of sodium chloride from about 800°C to about 600°C.
- Name the salt that is added (1 mk)
- Cryolite
- State why it is necessary to lower the temperature (1mk)
- Reduce the amount of energy used hence lower cost
- Name the salt that is added (1 mk)
- Explain why aqueous sodium chloride is not suitable as an electrolyte for the manufacture of sodium in the Downs process (2 mks)
- H+ will be preferentially discharged hence H2(g) will be produced instead of Na.
- Sodium metal reacts with air to form two oxides. Give the formulae of two oxides (1 mk)
Na2O(s)/Na2O2
- The above is a simplified diagram of the Downs Cell used for the manufacture of sodium. Study it and answer the questions that follow.
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 2 Questions and Answers - Moi Kabarak High School Mock 2020/2021.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students