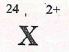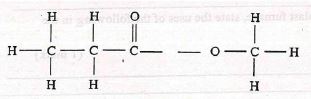CHEMISTRY
PAPER 1
TIME: 2 HOURS.
Instructions to candidates
- Answer ALL the questions in the spaces provided.
-
- A patient was given tablets with prescription 2 x 3 on the envelope. Clearly outline how the patient should take the tablets. (1 mark)
- Two samples of equal volumes of water were put in 250cm beaker and heated for 10 minutes. Sample,l registered a higher temperature than sample 2.
State the conditions under which flame 1 is produced in Bunsen burner.(1 mark)
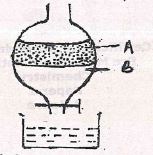
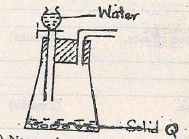
- The apparatus below was used to separate a mixture of liquid A and B
State two properties of the liquids that make it possible to separate them using such apparatus. (2 marks) - Describe how solid samples of salts can be obtained from a mixture of lead (II) chloride, sodium chloride and ammonium chloride. (3 marks
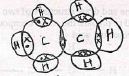
- An ion of element X is represented as
- Write electronic configuration of ion of x (1 mark)
- To which group does element x belong? (1 mark)
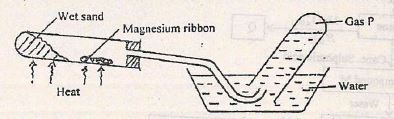
- The set-up below can be used to study the reaction of magnesium and steam.
- Name gas P. (1 mark)
- How would you expect copper to behave compared to magnesium in the combustion tube? (1 mark)
- Write the equation for the reaction between magnesium and steam (1 mark)
- An approximately x molar solution of potassium manganite (VII) solution was standardized against precisely 0.1M iron (II) ammonium sulphate ((NH4)3 Fe(SO4)2.6H2O] solution. 25.0cm3 of the solution of the iron (II) salt were oxidized by 24.15cm3 of the manganite (VII) solution. The equation of the reaction is:
MnO4(aq)- + 5Fe(aq)2+ + 8H(aq)+ → Mn(aq)2+ + 5Fe(aq)3+ + 4H2O(l)
What is the molarity of the potassium manganite (III) solution? (3 marks) - During extraction of iron in the blast furnace, state the uses of the following in the furnace.
- Molten slag. (1 mark)
- Waste gases leaving the furnace (1 mark)
- Limestone (1 mark)
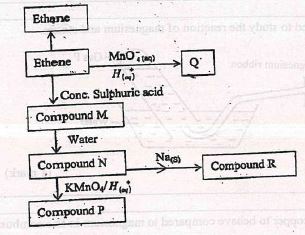
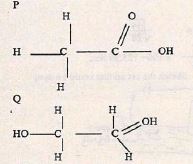
- The flow chart below gives some reactions starting with ethane. Study it and answer the questions that follow.
- Draw the structure of compounds
- (1 mark)
- (1 mark)
- Write the name of compound R. (1 mark)
- Draw the structure of compounds
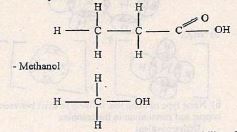
- Study the organic compound below:
- In which homologous series does the compound belong to? (1 mark)
- Name and draw the structures of two compounds that can be used to prepare the above compound. (3 marks)
-
- State one factor that can determine the stability of an atom (1 mark)
- Radioactive polonium - 216 decay as shown below.
216Po → 208Pb + M□ + nß
90 82
Find the value of M and n (2 marks) - If after 112 days, 1/16 of polonium remained, calculate the half-life of polonium (1 mark)
- A metal oxide has a formula M203.
- Write an equation to show how M form an ion (1 mark)
- Write the formula of the chloride of M. (1 mark)
- The thermodynamic equation for the formation of ammonia in the Haber process is
N2(g) + 3H2(g)2NH3(g), ΔH = -92kJ mol-1
State and explain one way in which the yield of ammonia can be increased. (2 marks) - A certain carbonate, JCO3, reacts with dilute hydrochloric acid according to the equation below.
JCO3 + 2HCl (aq) — → GCl(aq) + CO2(g) + H2O(l)
If 1g of the carbonate reacts completely with 20cm3 of 1M hydrochloric acid, calculate the relative atomic mass of J.
(C = 12,0= 16) (4 marks) -
- What is meant by the term solubility? (1 mark)
- The mass of a solution A is 120g. This solution has 8g of salt A dissolved in it. The solubility of this salt is 25g/100g of water at 30°C. 55g of salt A are added to the solution at 30°C. How much of salt A will remain undissolved. (2 marks)
-
- Using electrons in the outermost energy level, draw the dot (.) and cross (X) diagrams to represent bonding in.
- C2H6 (C= 6, H=1) (1 mark)
- NH4C1 (N = 7, H = 1, Cl = 17)(1 mark)
- The formula of a complex ion is [Cu(NH3)4]2+ name the type of bond that is likely to exist between copper and ammonia in the complex (1 mark)
- Using electrons in the outermost energy level, draw the dot (.) and cross (X) diagrams to represent bonding in.
-
- State Hess's law (1 mark)
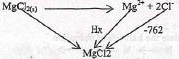
- Study the information below and answer the questions that follow.
MgCl2(s) → Mg(g)2+ + 2Cl(g)-, ΔH1 = -2487kJ mol-1
MgCl2(s) + (aq) → MgCl2(aq), ΔH2 = -5142kJ Mol-1
2Cl(g)-+ (aq) → 2Cl(aq)-, ΔH3 = -762kJ mol-1- Name the enthalpies H1 and H2 (2 marks)
- Determine the enthalpy for the reaction:
Mg(g)2+ + (aq) → Mg(g)2+ (2 marks)
-
- Give two reasons why carbon (IV) oxide is used as a fire extinguisher (1 mark)
- State: the function of tartaric acid in baking powder. (2 marks)
- When an electric current of 0.5A was passed through a molten chloride of J for 32 minutes and 10 seconds, a mass of 0.44g off J was deposited at the cathode. (IF = 96500C)
- Calculate the quantity of electricity used. (1 mark)
- Determine the value of x if the ion of metal J is represented as Jx+
(R.A.M of J= 14) (1 mark)
-
- What is meant by the term basicity of an acid? (1 mark)
- Describe briefly how potassium sulphate can be prepared using 50cm potassium hydroxide of 1M (3 marks)
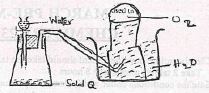
- The diagram below represents a set-up used to prepare oxygen gas.
- Name substance Q (1 mark)
- Complete the set up to show how oxygen gas is collected.(1mark)
- Write the equation for the reaction that occurs. (1 mark)
- The table below shows some solutions and their pH values
Which of the above solutionSolution pH value P 1.5 Q 6.0 R 14.0 S 8.0 - Is strongly basic. (1 mark)
- Reacts with sodium carbonate more vigorously (1 mark)
- Is ammonia solution (1 mark)
- In an experiment, a jar containing sulphur (IV) oxide was inverted over another jar containing hydrogen sulphide gas.
- State and explain the observation that was made. (2 marks)
- State two conditions necessary for the reaction to take place. (2 marks)
- Two reagents that can be used to prepare chlorine gas are potassium manganite (VII) and hydrochloric acid.
- Write an equation for the reaction. (1 mark)
- Give the formula of another reagent that can be used instead of potassium manganate (VII) (1 mark)
- Using an equation illustrate how chlorine bleach coloured substances. (1 mark)
-
- Distinguish between ionization energy and electron affinity. (2 marks)
- Explain why fluorine is more reactive than iodine. (2 marks)
- 280cm3 of nitrogen gas diffuse through a porous plug in 70 seconds. How long will it take 400cm of carbon (IV) oxide gas to diffuse through the same porous plug? (C = 12, 0= 16, N = 7). (3 marks)
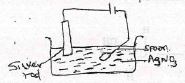
- An iron spoon was to be electroplated with silver. Sketch the set-up that could be used. (2 marks)
- Write the equation for decomposition of:
- Sodium nitrate. (1 mark)
- Copper (II) nitrate (1 mark)
MARKING SCHEME
- A patient was given tablets with prescription 2 x 3 on the envelope. Clearly outline how the patient should take the tablets. (1 mark)
- Take 2 tablets after every 8 hours
- Take 2 tablets after every 8 hours
- Two samples of equal volumes of water were put in 250cm beaker and heated for 10 minutes. Sample,l registered a higher temperature than sample 2.
State the conditions under which flame 1 is produced in Bunsen burner.(1 mark)- The flame should be produced when the air hole is open
- The flame should be produced when the air hole is open
- A patient was given tablets with prescription 2 x 3 on the envelope. Clearly outline how the patient should take the tablets. (1 mark)
- The apparatus below was used to separate a mixture of liquid A and B
State two properties of the liquids that make it possible to separate them using such apparatus. (2 marks)- Difference in densities
- They are immiscible
- Describe how solid samples of salts can be obtained from a mixture of lead (II) chloride, sodium chloride and ammonium chloride. (3 marks)
- Heat to sublime ammonium chloride
- Add water to dissolve sodium chloride
- Filter the residue is lead (II) chloride .
- Evaporate the filtrate (sodium chloride solution) to obtain sodium chloride solid.
- An ion of element X is represented as
- Write electronic configuration of ion of x (1 mark)
- 2:8
- 2:8
- To which group does element x belong? (1 mark)
- Group 2 (II)
- Group 2 (II)
- Write electronic configuration of ion of x (1 mark)
- The set-up below can be used to study the reaction of magnesium and steam.
- Name gas P. (1 mark)
- Hydrogen
- Hydrogen
- How would you expect copper to behave compared to magnesium in the combustion tube? (1 mark)
- Copper would not react with steam
- Copper would not react with steam
- Write the equation for the reaction between magnesium and steam (1 mark)
- Mg(s) + H2O(g) → MgCl(s) + H2(g)
- Mg(s) + H2O(g) → MgCl(s) + H2(g)
- Name gas P. (1 mark)
- An approximately x molar solution of potassium manganite (VII) solution was standardized against precisely 0.1M iron (II) ammonium sulphate ((NH4)3 Fe(SO4)2.6H2O] solution. 25.0cm3 of the solution of the iron (II) salt were oxidized by 24.15cm3 of the manganite (VII) solution. The equation of the reaction is:
MnO4(aq)- + 5Fe(aq)2+ + 8H(aq)+ → Mn(aq)2+ + 5Fe(aq)3+ + 4H2O(l)
What is the molarity of the potassium manganite (III) solution? (3 marks)
Mm = ?
Vm= 24.15cm3
MFe-0.1M
VFe = 25cm3
Mole of Fe2+ = 0.1mole = 1000cm3
? = 25cm
Mole ratio 1:5= 0.0025
moles Moles of MnO4 = 0.0025 = 0.005 moles - During extraction of iron in the blast furnace, state the uses of the following in the furnace.
- Molten slag. (1 mark)
- Protect the hot iron from being re-open
- Protect the hot iron from being re-open
- Waste gases leaving the furnace (1 mark)
- used to preheat the air between that time base of the furnace
- Limestone (1 mark)
- Decompose to CaO which combine with unwanted silica forming slage
- Molten slag. (1 mark)
- The flow chart below gives some reactions starting with ethane. Study it and answer the questions that follow.
- Draw the structure of compounds
- (1 mark)
- (1 mark)
- Write the name of compound R. (1 mark)
- Sodium ethoxide
- Sodium ethoxide
- Draw the structure of compounds
- Study the organic compound below:
- In which homologous series does the compound belong to? (1 mark)
- Ester
- Ester
- Name and draw the structures of two compounds that can be used to prepare the above compound. (3 marks)
- propanoic acid
- methanol
- In which homologous series does the compound belong to? (1 mark)
-
- State one factor that can determine the stability of an atom (1 mark)
- N/P ratio
- Amount of energy released when heat collide with protons in the nucleus
- Radioactive polonium - 216 decay as shown below.
216Po → 208Pb + M□ + nß
90 82
Find the value of M and n (2 marks)- 216 - 208 + 4 +0
4m - 216 - 208
4m = 8/4=2
90 - 82 + 2m + -n
- 216 - 208 + 4 +0
- If after 112 days, 1/16 of polonium remained, calculate the half-life of polonium (1 mark)
- 1 → 1/2 → 1/4 → 1/8 → 1/16
4 half lives → 112 days
1 half life → ?
=1 x 112/4
=28 days
- 1 → 1/2 → 1/4 → 1/8 → 1/16
- State one factor that can determine the stability of an atom (1 mark)
- A metal oxide has a formula M203.
- Write an equation to show how M form an ion (1 mark)
- M(g) - 3e- → M(g)3+
- M(g) → M(g)3+ + 3e-
- Write the formula of the chloride of M. (1 mark)
- MCl3
- MCl3
- Write an equation to show how M form an ion (1 mark)
- The thermodynamic equation for the formation of ammonia in the Haber process is
N2(g) + 3H2(g)2NH3(g), ΔH = -92kJ mol-1
State and explain one way in which the yield of ammonia can be increased. (2 marks)- Increase in pressure
- Withdraw of NH3(g) decrease in concentration of NH3(g) favours towards reaction
- Use of low temperature RXA of exothermic decreases in temperature forms forward reaction
- Addition of H3/N2 increase in reaction of mxns favour forward reaction
- A certain carbonate, JCO3, reacts with dilute hydrochloric acid according to the equation below.
JCO3 + 2HCl (aq) — → GCl(aq) + CO2(g) + H2O(l)
If 1g of the carbonate reacts completely with 20cm3 of 1M hydrochloric acid, calculate the relative atomic mass of J.
(C = 12,0= 16) (4 marks)- moles of HCl used = 1 x 20
1000 =0.02 moles
CaCO3 : HCI
1 2
Moles of CaCO3 used = 1/2 x 0.02 moles = 0.01 moles
0.01 mole = 1g
1 mole =?
=1 x 1
0.01 = 100g
Ca + 12 + 16 + 3 = 100
Ca = 100-60
Ca=40
- moles of HCl used = 1 x 20
-
- What is meant by the term solubility? (1 mark)
- Solubility is the mass of a substance that can ndissolve per 100g of water
- Solubility is the mass of a substance that can ndissolve per 100g of water
- The mass of a solution A is 120g. This solution has 8g of salt A dissolved in it. The solubility of this salt is 25g/100g of water at 30°C. 55g of salt A are added to the solution at 30°C. How much of salt A will remain undissolved. (2 marks)
- 100g of water = 25g
112g of water = ?
112g x 25g
100g
=28g
Un dissolved salt
- (8 + 55) - 28 = 35g
- 100g of water = 25g
- What is meant by the term solubility? (1 mark)
-
- Using electrons in the outermost energy level, draw the dot (.) and cross (X) diagrams to represent bonding in.
- C2H6 (C= 6, H=1) (1 mark)
- NH4C1 (N = 7, H = 1, Cl = 17)(1 mark)
- C2H6 (C= 6, H=1) (1 mark)
- The formula of a complex ion is [Cu(NH3)4]2+ name the type of bond that is likely to exist between copper and ammonia in the complex (1 mark)
- Dative covalent
- Using electrons in the outermost energy level, draw the dot (.) and cross (X) diagrams to represent bonding in.
-
- State Hess's law (1 mark)
- Enthalpy change of a reaction is the same regardless of the rate followed as long as the reactants and products are the same.
- Enthalpy change of a reaction is the same regardless of the rate followed as long as the reactants and products are the same.
- Study the information below and answer the questions that follow.
MgCl2(s) → Mg(g)2+ + 2Cl(g)-, ΔH1 = -2487kJ mol-1
MgCl2(s) + (aq) → MgCl2(aq), ΔH2 = -5142kJ Mol-1
2Cl(g)-+ (aq) → 2Cl(aq)-, ΔH3 = -762kJ mol-1- Name the enthalpies H1 and H2 (2 marks)
- H1 - lattice energy
- H2 - Hydration energy of MgCl2 solution
- Determine the enthalpy for the reaction:
Mg(g)2+ + (aq) → Mg(g)2+ (2 marks)
-5142=-2489 +Hx+ -762 x 2
Hx = -5142 +2489769 = -1893kj/mol
- Name the enthalpies H1 and H2 (2 marks)
- State Hess's law (1 mark)
-
- Give two reasons why carbon (IV) oxide is used as a fire extinguisher (1 mark)
- Denser than air
- Does not support combustion
- State: the function of tartaric acid in baking powder. (2 marks)
- Reacts with NaHOO, to produce CO, which makes the dough to rise
- Reacts with Na C0, formed when NaHCO, is heated hence neutralize Na Co, in the dough
- Give two reasons why carbon (IV) oxide is used as a fire extinguisher (1 mark)
- When an electric current of 0.5A was passed through a molten chloride of J for 32 minutes and 10 seconds, a mass of 0.44g off J was deposited at the cathode. (IF = 96500C)
- Calculate the quantity of electricity used. (1 mark)
- Q = it = 0.5 x 1930 sec = 965c
- Determine the value of x if the ion of metal J is represented as Jx+
(R.A.M of J= 14) (1 mark)- Jx + xC. J(s) 445
965c = 0.44g
?= 44g
=96500C
X x 96500 = 965500
96500 96500
X = 1 Therefore charge +1
- Jx + xC. J(s) 445
- Calculate the quantity of electricity used. (1 mark)
-
- What is meant by the term basicity of an acid? (1 mark)
- It is the number of replaceable hydrogen atoms in an acid
- It is the number of replaceable hydrogen atoms in an acid
- Describe briefly how potassium sulphate can be prepared using 50cm potassium hydroxide of 1M (3 marks)
- Mix/react 50cm3 of 0.5M H2SO4 or 25cm3 of 1M H2SO4 to obtain a neutral solution of K2SO4
- Hear to evaporate some water
- Cool slowly to crystallize the solution
- What is meant by the term basicity of an acid? (1 mark)
- The diagram below represents a set-up used to prepare oxygen gas.
- Name substance Q (1 mark)
- Na2O2
- Na2O2
- Complete the set up to show how oxygen gas is collected.(1mark)
- Write the equation for the reaction that occurs. (1 mark)
- 2Na2O2 + 2H2O(l) → 4NaOH(aq) + O2
- Name substance Q (1 mark)
- The table below shows some solutions and their pH values
Which of the above solutionSolution pH value P 1.5 Q 6.0 R 14.0 S 8.0 - Is strongly basic. (1 mark)
- R/14.0
- R/14.0
- Reacts with sodium carbonate more vigorously (1 mark)
- R/1.5
- R/1.5
- Is ammonia solution (1 mark)
- S/8.0
- S/8.0
- Is strongly basic. (1 mark)
- In an experiment, a jar containing sulphur (IV) oxide was inverted over another jar containing hydrogen sulphide gas.
- State and explain the observation that was made. (2 marks)
- Yellow solid is formed
- SO2(g) is reduced by H2S to S
- State two conditions necessary for the reaction to take place. (2 marks)
- Jars should be moist
- The jar with the denser has should be placed on top of the jar with the gas.
- State and explain the observation that was made. (2 marks)
- Two reagents that can be used to prepare chlorine gas are potassium manganite (VII) and hydrochloric acid.
- Write an equation for the reaction. (1 mark)
- 2KMnO4(s) + 16HCl(aq) → KCl + 2MnCl(g) + 8H2O(l) + 5Cl2
- 2KMnO4(s) + 16HCl(aq) → KCl + 2MnCl(g) + 8H2O(l) + 5Cl2
- Give the formula of another reagent that can be used instead of potassium manganate (VII) (1 mark)
- MnO2
- MnO2
- Using an equation illustrate how chlorine bleach coloured substances. (1 mark)
- Cl2(g) + dye + H2O(l) → 2HCl(aq) + dye - O
- Write an equation for the reaction. (1 mark)
-
- Distinguish between ionization energy and electron affinity. (2 marks)
- Ionization energy is the energy required to remove an electron from a gaseous atom/ion
- Electron affinity is the energy required to add an electron to a gascous atom
- Explain why fluorine is more reactive than iodine. (2 marks)
- Reactivity of halogens increase up the gp due to the decreased number of energy levels/ decrease in atomic radius
- Distinguish between ionization energy and electron affinity. (2 marks)
- 280cm3 of nitrogen gas diffuse through a porous plug in 70 seconds. How long will it take 400cm of carbon (IV) oxide gas to diffuse through the same porous plug? (C = 12, 0= 16, N = 7). (3 marks)
- RN2 = 230
70
=4cm/sec
RCO2 = 400/t
4
400/t = √44/28
t= √44/28 x 100 = 125.36 sec.
- RN2 = 230
- An iron spoon was to be electroplated with silver. Sketch the set-up that could be used. (2 marks)
- Write the equation for decomposition of:
- Sodium nitrate. (1 mark)
- 2NaNO3(s) → 2NaNO2(s) + O2(g)
- 2NaNO3(s) → 2NaNO2(s) + O2(g)
- Copper (II) nitrate (1 mark)
- Cu(NO3)2(s) → 2CuO(s) + 4NO2(g) +O2(g)
- Sodium nitrate. (1 mark)
Download Chemistry Paper 1 Questions and Answers - Mang'u Mock 2020 Exam.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students