INSTRUCTIONS TO CANDIDATES
- Answer ALL the questions in the spaces provided in the question paper.
- All working MUST be clearly shown where necessary.
- Mathematical tables and silent electronic calculators may be used.
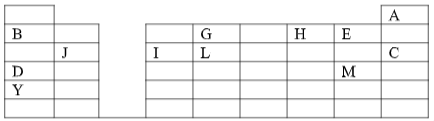
- The grid given below represents part of the periodic table. Study it and answer the questions that follow. The letters are not the actual symbols of the elements.
- What name is given to the family of elements to which A and C belong? ( 1 mark )
- Write the chemical formula of the sulphate of element D. ( 1 mark )
- Which letter represents the most reactive ( 2 marks )
- Metal
- Non-metal
- Name the bond formed when B and H react. Explain your answer. ( 2 marks )
- Select one element that belong to period 4. ( 1 mark )
- Ionic radius of element E is bigger than the atomic radius. Explain. ( 2 marks )
- The electron configuration of a divalent anion of element N is 2.8.8. Induce the Position of element N on the periodic table drawn above. ( 1 mark )
- The oxide of G has a lower melting point than the oxide of L. Explain. ( 1 mark )
- How do the atomic radii of I and C compare. Explain. ( 2 marks )
- Explain the trend in the 1st ionization energies of the elements J, I and L.(1mark )
-
- define the following terms
- Saturated solution(1mk)
- Fractional crystallization(1mk)
- Solubility of salt X and Y were determined at different temperatures as shown in the following data.
Temperature (ºC) 0 20 40 60 80 100 Solubility of 100g of water X 12 30 75 125 185 250 Y 15 20 30 45 65 80 - On the grid provided, plot a graph of solubility (vertical axis) against temperature. (4mks)
- From the graph determine the solubility of each at 50ºC.
X ……………………………………………………….. (1mk)
Y ………………………………………………………… (1mk) - At what temperature was the solubility of both salts equal. (1mk)
- What is permanent hardness of water? (1mk)
- define the following terms
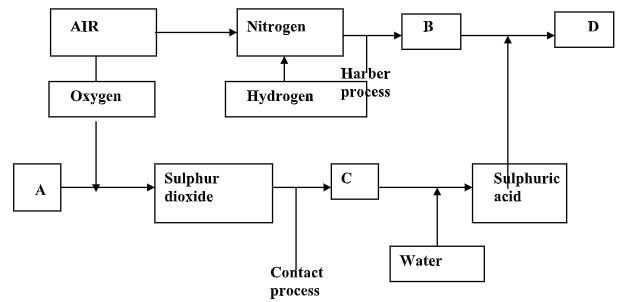
- The flow chart below illustrates two industrial processes. Haber and contact processes each with air as one of the starting materials and other chemical reactions.
-
- Give the name of the process by which air is separated into oxygen and nitrogen. (1 mk)
- Apart from oxygen and nitrogen gas produced from process a(i) name any other gas produced in the process above. (1 mk)
- Name the substances which are represented by the letter. (4 mks)
A……………………………..
B……………………………………..
C …………………………………….
D……………………………………… - Name the catalyst used in;
- The Haber process (1 mk)
- The contact process (1 mk)
- Explain the role of the catalyst in both the Haber and contact process. (2 mks)
-
- Write a balanced equation for formation of compound D. (1 mk)
- Calculate the percentage by mass of nitrogen present in compound D
(N = 14.0, H = 1.0, S = 32.0, O = 16.0) (2 mks) - Give one use of compound D. (1 mk)
-
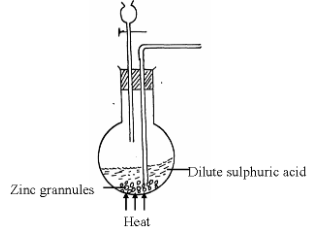
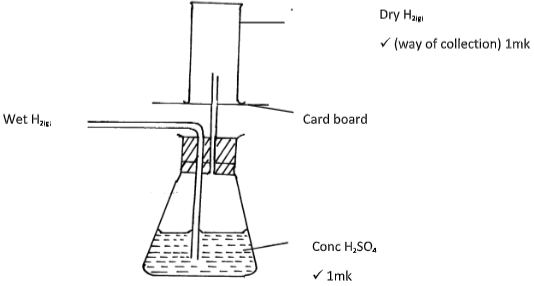
- A student set-up the arrangement below to prepare and collect dry hydrogen gas
- Identify two errors from the section of the arrangement shown above (2mks)
- Complete the diagram to show how dry hydrogen gas can be collected. (2mks)
-
- Explain why hydrogen was collected by the method shown above (1mk)
- Write a balanced chemical equation for the reaction that takes place when hydrogen gas is burnt in air. (1mk)
- Determine the relative atomic mass of zinc, given that when 6.54g of zinc was used, 2.4litres of hydrogen gas was produced. (Molar gas volume = 24 litres) (3mks)
- State any two non-industrial uses of hydrogen gas (2mks)
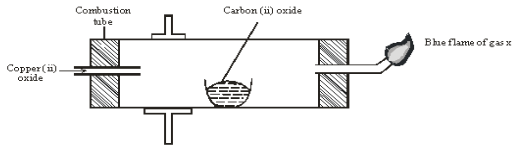
- The diagram below shows an experiment set-up to investigate a property of carbon (ii) oxide. Study it and answer the questions that follow.
- Name one condition that is missing in the set up that must be present if the experiment to proceed. 1mark
- If the experiment was carried out properly. What observation would be made in the combustion tube? 1mark
- Give an equation for the reaction that occurs in the combustion tube. 1 mark
- Give an equation for the reaction that takes place as gas x burns. 1 marks
- Why is it necessary to burn gas x? 1mk
- Name the reducing and oxidizing agent. 2marks
- Reducing agent
- Oxidising agent
- Identify any other substance that would have the same effect on copper (ii) oxide as carbon (ii) oxide. 1mark
- What would happen if copper (ii) oxide was replaced with sodium oxide? Explain 2mark
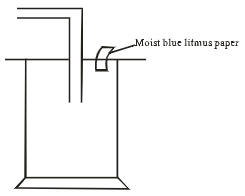
- Dry chlorine was collected using the set up below.
- Name a suitable drying agent for chlorine gas? (1mark)
- State one property of chlorine gas which facilitates this method of collection. 1mark
- State the observations made on the moist blue litmus paper. (2marks)
- Chlorine gas was bubbled through distilled water. With aid of an equation show the formation of chlorine water. (1mark)
- Write the formula of the compounds formed when chlorine gas reacts with warm dry phosphorous. (2marks)
- Chlorine gas is mixed with moist hydrogen sulphide gas, state and explain the observations (2marks)
- Give one use of chlorine gas. 1mark
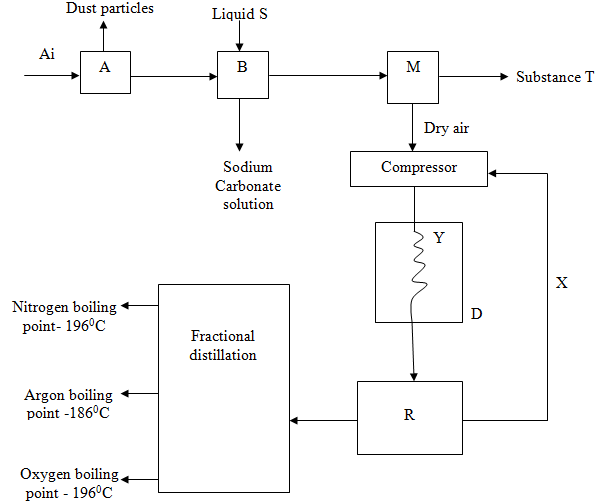
- Fractional distillation of air is used in the industrial manufacture of oxygen. The diagram below shows the process.
- What processes are taking place in chamber A,B,M and D 2marks
- Name;
- Liquid S(1mk)
- Substance T(1mk)
- Explain why part Y in chamber D is curved? 1mark
- Give two industrial uses of oxygen gas? (2marks)
- In the laboratory preparation of oxygen, manganese (iv) oxide and hydrogen peroxide are used. Write an equation to show how oxygen gas is formed. 1mark
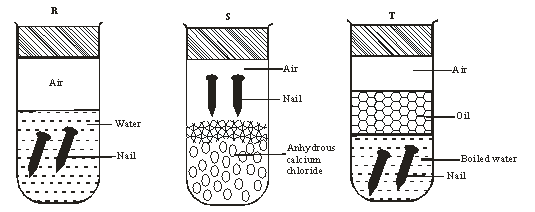
- An investigation was carried out using the set-up below. Study it and answer the questions that follow.
- State and explain what will happen in the three test-tubes R, S and T after seven days. 2marks
- Give one reason why some metals are electroplated. 1mark

MARKING SCHEME
-
- Noble gases √1
- D2SO4 √1
-
- Y √1
- E √1
- Ionic bond √1 – Because B reacts by losing an electron (s) which are gained by H. √1 accept transfer of electrons from a metal to non metal
- D//M √1 Any ½ mark each
- Because E reacts by gaining an extra electron which reduces √1 the electrostatic pull by the positive nucleus making the ionic radius increase. Or incoming electron causes increased repulsion wtte
- At Period III Group IV
- Because of the increase in the strength of the molecular bonds in the oxide of L as compared to that of G. √1 w.t.t.e
- C has a smaller atomic √1 radius than I because of the increase in the strength of the Nucleur foce of attraction in C as the number of protons increase √1 w.t.t.e
- 1st ionization energies increases from J – L across the period due to addition of an extra proton in the nucleus increasing the attraction of the valency electrons √
-
-
- A solution that cannot dissolve any more of the solute at that particular temperature. ✔ 1mk
- Scientific technique used to separate substances due to their differences in their crystallization temperature. ✔ 1mk or w.t.t.e
-
- on the scanned graph
- x=100g/100ml, y=40g/100ml
- 5°c
- type of hardness that cannot be removed by boiling
-
-
-
- Fractional distillation✔ 1mk
- Argon//neon/xenon//krypton✔ 1mk
-
- A Sulphur✔1mk
- B Ammonia gas✔1mk
- C sulphur (vi) oxide✔1mk
- D Ammonium sulphate✔1mk
-
- Finely divided iron✔1mk
- Vanadium (v) oxide✔1mk
- The catalysts fasten✔1mk the Haber & contact processes by lowering the activation energy✔1mk of the reactions//the rate of production is increased.
-
- H2SO4(aq) + 2NH3(g) → (NH4)2SO4(aq)✔1mk
- Formula mass of (NH4)2SO4 = 2(14+4) + 32 + 4(16)
= 132grams✔ ½ mk
% of N = 28/132 × 100 ✔1mk
= 21.212%✔ ½ mk - Use as a fertilizer✔1mk
-
-
-
- The outlet delivery tube should not dip into the Zinc/dilute Sulphuric acid mixture in the round buttoned flask. ✔ 1mk
- The use of heat is not required ✔ 1mk
-
-
- It is denser than air ✔1 mk
- H2(g) + ½ O2(g) → H2O(g)✔ balancing½ mark states ½ mark
- Zn2(s) + H2SO4(aq) → ZnSO4(aq) + H2(g) balancing ½ mk states ½ mk
1vol 1 vol 1vol
(6.54/R) (2.4/24)
Therefore, (6.54/R) = 2.4/2 ✔1mk Where R = R.A.M of Zinc
R = 24 × 6.54
2.4
Or R = 65.4 ✔ 1mk -
- H2(g) is used in balloons by meteorologists ✔1mk
- It is used as rocket fuel ✔ 1mk
-
-
- Heating copper (ii) oxide √1mk
- Black solid would turn brown √1mk
- CuO(s) + CO(g) → Cu(s) + CO2(g) √1 ½ mk
- 2CO(g) + O2(g) → 2CO2(g) √1 ½ mk
- It is poisonous √1mk
-
- Reducing agent - Carbon(ii) oxide √1mk
- Oxidisingagent -Copper (ii) oxide √1mk
- Hydrogen / ammonia gas (Any one) √1mk
- There would be no observable change √1mk. This is because sodium is higher than carbon in the reactivity series and therefore has higher affinity of oxygen √1mk
-
- Concentrated sulphuric (vi) acid √1mk
- It is denser than air √1mk
-
- It turns red then white. √1mk
- It turns white / it gets bleached √1mk
- Cl2(g) + H2O(l) → HOCl(aq) + HCl(aq) √1mk
-
- PCl3 √1mk
- PCl5 √1mk
-
- A yellow deposit of sulphur is formed / seen √1mk
- Chlorine oxidizes sulphideions to solid sulphur √1mk
-
- Manufacture of hydrochloric acid √1mk
- Manufacture of bleaching agents such as chlorate used in the cotton and paper industries
- Chlorine is used in the treatment of water and sewage plants
- Manufacture of chloroform as an anaesthetic
- Manufacture of solvents such as trichloroethane
Any one
-
- A - Filtration √1 ½ mk
B - Absorption √1 ½ mk
M - Isolation of water √1 ½ mk
D - Cooling √1 ½ mk -
- Liquids – NaOH (aq) / KOH (aq) √1mk
- Substance T – Ice / water √1mk
- To increase surface area forcooling √1 mk
-
- Oxygen is used to remove impurities during steel making √1 mk
- Is used in cutting and welding of metals √1 mk
- 2H2O2(l) MnO2(S) 2H2O(l)+ O2(g) √1 mk
-
- R -Rusting occurred √1 ½ mk because of air and water being present √½ mk
S - No rusting √½ mk Water is absent √½ mk
T - No rusting √ ½ mk Air is absent √½ mk -
- To prevent rusting √1mk
- To increase aesthetic value of the metal
- R -Rusting occurred √1 ½ mk because of air and water being present √½ mk
- A - Filtration √1 ½ mk
Download Chemistry Paper 2 Questions and Answers - Bungoma Diocese Mock Exams 2021/2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students