INSTRUCTIONS TO CANDIDATES
- Answer all questions in the spaces provided
- KNEC mathematical tables and silent electronic calculators may be used for calculations.
- All workings must be clearly shown where necessary.
- A certain element Y has atomic number 15 and mass number of 31.
- How many electrons are in this element? (1mk)
- Write the electron arrangement of the ion formed by element Y. (1mk)
- How would the atomic size of the above element compare with another atom X whose atomic number is 11 and mass number 23? Explain. (2mks)
- Explain why the pH of 1.0 M hydrochloric acid is 2.0 while that of 1.0M ethanoic acid is 5.0. (2mks)
- Ethanedioic acid (COOH)2 is used instead of methanoic acid (HCOOH) to prepare carbon (II) oxide in the laboratory. It gives equal volume of carbon (II) oxide and carbon (IV) oxide.
- If water is one of the products write an equation for the dehydration of elthanedioic acid. (1mk)
- How can pure carbon (II) oxide be obtained from the mixture of the two gases? (2mks)
-
- State the Charles’ law. (1mk)
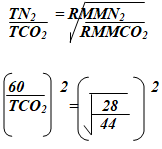
- A volume of 120cm3 of nitrogen gas diffused through a membrane in 40 seconds, how long will 180cm3 of carbon (IV) oxide take to diffuse through the same membrane? (2mks)
- A solution of chlorine in tetrachloromethane turns colourless when propene gas is bubbled through it.
- What type of reaction takes place? (1mk)
- Write an equation for the above reaction. (1mk)
- State one use of ethane gas. (1mk)
- Study the information in the table below then answer the question that follow.
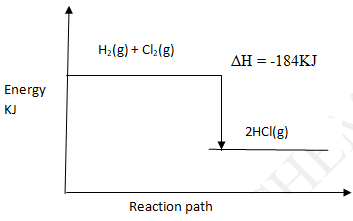
Bond Bond energy (KJmol-1) H – H
Cl –Cl
H – Cl435
243
431- Calculate the enthalpy change for the following reaction. (2mks)
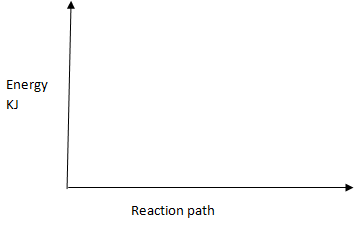
H2(g) + Cl2(g) → 2HCl (g) - On the axis given below draw an energy level diagram for the reaction above. (1mk)
- Calculate the enthalpy change for the following reaction. (2mks)
- 22.2cm3 of sodium hydroxide solution, containing 4.0 g per litre of sodium hydroxide were required for complete neutralization of 0.1g of a dibasic acid. Calculate the relative formula mass of the dibasic acid. (Na = 23.0, O= 16.0, H= 1.0) (3mks)
- The melting and boiling point of molecular substances increase with increase in relative molecular mass. Explain why water with a lower relative molecular mass of 18 has a higher boiling point of 100°C than hydrogen sulphide with relative molecular mass of 34 and a boiling point of -61°C. (2mks)
- In an experiment to determine the solubility of solid Y in water at 30°C the following results were obtained:
Mass of evaporating dish = 26.2.
Mass of evaporating dish + saturated solution = 42.4g
Mass of evaporating dish + dry solid Y = 30.4g
Using the information, determine the solubility of solid Y at 30°C in grams per 100g. (2mks) - A, B, C and D are dyes present in a mixture. C is more soluble than B. A is more soluble than C while D is the least soluble in a given solvent. Draw a round paper chromatogram showing how they would appear when separated using the solvent. (2mks)
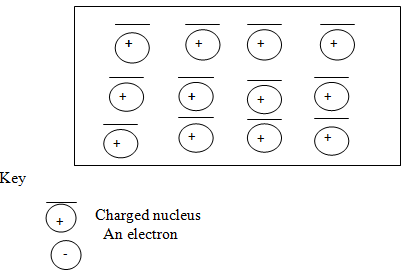
- The diagram below shows a section of a model of the structure of element T.
- State the bonding type that exists in element T. (1mk)
- In which group of the periodic table does element T belong? Give a reason. (1mk)
-
- Determine the values of the scalars X and Y in the nuclear equation shown below.
- State one application of this type of reaction. (1mk)
- State one danger associated with exposure of human beings to radioactive substances. (1mk)
- Determine the values of the scalars X and Y in the nuclear equation shown below.
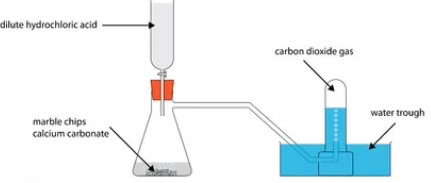
- The diagram below shows an incomplete set up of the laboratory preparation of dry carbon (IV) oxide. Complete it. (2mks)
- Write down the property of concentrated sulphuric (VI) acid shown in the following reaction. (2mks)
- H2SO4(l)
CuSO4. 5H2O(s)CuSO4(s) +5H2O(l)
Blue White - C(s) + 2H2SO4(I) → CO2(g) + 2H2O(I) + 2SO2(g)
- H2SO4(l)
- When excess chlorine gas is bubbled through dilute sodium hydroxide solution, the resulting solution acts as a bleaching agent.
- Write an equation for the reaction between chlorine gas and sodium hydroxide solution. (1mk)
- Explain how the resulting solution acts as a bleaching agent. (2mks)
- An element P has a relative atomic mass of 88. When a current of 0.5 ampheres was passed through the fused chloride for 32.16 minutes, 0.44g of P were deposited at the cathode. Determine the charge on an ion of P. (1 F = 96500 coulombs) (3mks)
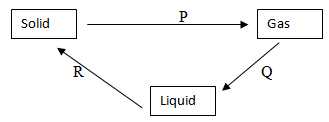
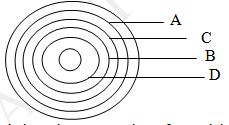
- Matter exists in three states which can be related as shown in the diagram below.
- Name processes P and R. (2mks)
- Explain whether process Q is exothermic or endothermic. (1½ mks)
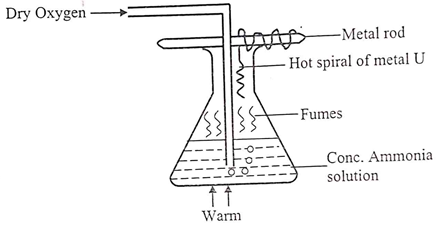
- Study the diagram below.
- Give the most likely identity of metal U. (1mk)
- State two observations made in the conical flask. (2mks)
- Write an equation for the reaction which took place between ammonia and oxygen inside the flask. (1mk)
- In an experiment, soap solution was added to three separate samples of water. The table below shows the volumes of soap solution added in order to form lather with 1000cm3 of each samples before and after boiling.
Sample I Sample II Sample III Volume of soap added to unboiled sample (cm3) 27.0 3.0 10.6 Volume of soap added after boiling the sample (cm3) 27.0 3.0 3.0 - Identify the sample that was likely to be soft water. Explain. (1mk)
- Explain the change in the volume of soap solution in sample III. (1mk)
- Give one disadvantage of hard water. (1mk)
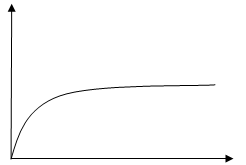
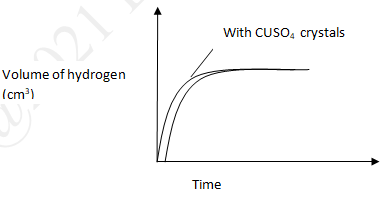
- In an experiment to monitor the rate of reaction of magnesium and hydrochloric acid a student recorded the volume of hydrogen produced at regular time intervals and obtained the graph shown below.
- On the same set of axes sketch the curve expected if the experiment is repeated with a few crystals of copper (II) sulphate added to the reactants. (1mk)
- Explain the shape of your curve. (1mk)
- State the factor that can increase the rate of a reaction. (1mk)
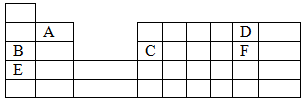
- The grid below is a section of the periodic table. Study it and answer the questions that follow.
- State the name given to the family of B and E. (1mk)
- Identify the most reactive metal. (1mk)
- Which type of bond exists in the compound formed by A and F. Explain. (1 ½ mks)
-
- Write the formula of the chief ore (bauxite) from which aluminum is extracted. (1mk)
- Explain the role of molten cryolite in aluminum smelting. (1mk)
- Aluminum does not apparently react with dilute nitric (V) acid to liberate hydrogen gas. Explain. (1mk)
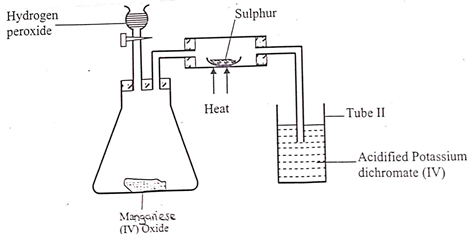
- Study the diagram below.
- State the role of manganese (IV) oxide in the set up shown above. (1mk)
- State and explain the observation made in tube II. (2mks)
- Two manila papers were placed at different levels of a non- luminous flame. Paper A was placed at the lowest part of the flame, while B was placed at the top.
- Indicate the observations made on each manila paper. (1mk)
- Explain the observation made on paper A. (1mk)
- Starting with 50cm3 of 2.8M sodium hydroxide, describe how a sample of pure sodium sulphate crystals can be prepared. (3mks)
- In an experiment to determine the percentage of magnesium hydroxide in an anti-acid a solution containing 0.5g of the anti-acid was neutralized by 23.0cm3 of 0.10M hydrochloric acid. Given the relative formula mass of magnesium hydroxide is 58. Calculate the:
- Mass of magnesium hydroxide in the anti-acid. (2mks)
- Percentage of magnesium hydroxide in the anti-acid. (1mk)
- Study the standard electrode potentials in the table below and answer the questions that follow.
EѲvolts
Cu2+(aq) + 2e− → Cu(s) + 0.34
Mg2+(aq) + 2e− → Mg(s) −2.38
Ag+(aq) + e− → Ag(s) +0.80
Ca2+(aq) + 2e− → Ca(s) −2.87- Which of the metals is the strongest reducing agent? (1mk)
- What observations will be made if a silver coin is dropped into an aqueous solution of copper (II) sulphate? Explain. (2mks)
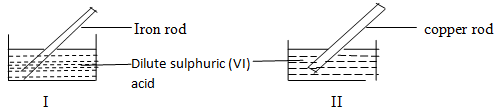
- A student used the figure below to investigate the action of dilute sulphuric (VI) acid on some metals. Beaker I and II contained equal volumes of dilute sulphuric (VI) acid. To beaker I, a clean iron rod was dipped and to beaker II, a clean copper rod was dipped.
- Describe the observations made in each beaker.
- Beaker I (1mk)
- Beaker II (1mk)
- Explain observations in (a) above. (1mk)
- Describe the observations made in each beaker.

MARKING SCHEME
- A certain element Y has atomic number 15 and mass number of 31.
- How many electrons are in this element? (1mk)
- 15
- Write the electron arrangement of the ion formed by element Y. (1mk)
- 2.8.8
- How would the atomic size of the above element compare with another atom X whose atomic number is 11 and mass number 23? Explain. (2mks)
- The atomic size of Y is smaller than that of X because Y has more protons than X hence the outer most electrons are more tightly held.
- How many electrons are in this element? (1mk)
- Explain why the pH of 1.0 M hydrochloric acid is 2.0 while that of 1.0M ethanoic acid is 5.0. (2mks)
- Hydrochloric acid is a strong acid and is completely dissociated giving a high concentration of H+ ions while ethanoic acid is only partially dissociated, being a weak acid hence its pH is 5.0.
- Ethanedioic acid (COOH)2 is used instead of methanoic acid (HCOOH) to prepare carbon (II) oxide in the laboratory. It gives equal volume of carbon (II) oxide and carbon (IV) oxide.
- If water is one of the products write an equation for the dehydration of elthanedioic acid. (1mk)
conc H2SO4(l)
(COOH)2(s)CO2(g) + CO(g) + H2O(l)
- How can pure carbon (II) oxide be obtained from the mixture of the two gases? (2mks)
- By passing the mixture through a concentrated solution of sodium hydroxide/potassium hydroxide. Carbon (IV) oxide is absorbed leaving behind the carbon (II) oxide.
- If water is one of the products write an equation for the dehydration of elthanedioic acid. (1mk)
-
- State the Charles’ law. (1mk)
- The volume of a fixed mass of a gas is directly proportional to its absolute temperature at a constant pressure.
- A volume of 120cm3 of nitrogen gas diffused through a membrane in 40 seconds, how long will 180cm3 of carbon (IV) oxide take to diffuse through the same membrane? (2mks)
120cm3 of N2 diffuse in 40 sec
180cm3 of N2 ?
= 180 x 40
120
= 60 sec
3600 = 28
TCO2 44
(TCO2)2 x 28 = 3600 x 44
28
√(TCO2)2 = √5657.1429
TCO2 = 75.21 sec
- State the Charles’ law. (1mk)
- A solution of chlorine in tetrachloromethane turns colourless when propene gas is bubbled through it.
- What type of reaction takes place? (1mk)
- Addition reaction
- Write an equation for the above reaction. (1mk)
CH3CH = CH2(g) + Cl2(g) → CH3CHClCH2Cl - State one use of ethane gas. (1mk)
- Used as fuel
- What type of reaction takes place? (1mk)
- Study the information in the table below then answer the question that follow.
Bond Bond energy (KJmol-1) H – H
Cl –Cl
H – Cl435
243
431- Calculate the enthalpy change for the following reaction. (2mks)
H2(g) + Cl2(g) → 2HCl(g)
ΔH= Bondbreaking + bond formation
−435 + 243 + 2 (−431)
= 678 – 862
= −184KJ - On the axis given below draw an energy level diagram for the reaction above. (1mk)
- Calculate the enthalpy change for the following reaction. (2mks)
- 22.2cm3 of sodium hydroxide solution, containing 4.0 g per litre of sodium hydroxide were required for complete neutralization of 0.1g of a dibasic acid. Calculate the relative formula mass of the dibasic acid. (Na = 23.0, O= 16.0, H= 1.0) (3mks)
Molarity of NaOh = 4 = 0.1M
40
Moles of NaOH in 22.2cm3 = 0.1 x 22.2
1000
= 0.00222 moles
Reacting mole ratio
NaOH : H2X
2 : 1
Moles of dibasic acid, H2X = 0.00222 x ½
= 0.00111 moles
RFM of H2x = mass = 0.1
Moles = 0.00111
= 90.09
RFM of H2X = 90 - The melting and boiling point of molecular substances increase with increase in relative molecular mass. Explain why water with a lower relative molecular mass of 18 has a higher boiling point of 100°C than hydrogen sulphide with relative molecular mass of 34 and a boiling point of −61°C. (2mks)
- In water the molecules are held together by hydrogen bonds while molecules in hydrogen sulphide are held by weak van der waals forces.
- In an experiment to determine the solubility of solid Y in water at 30°C the following results were obtained:
Mass of evaporating dish = 26.2.
Mass of evaporating dish + saturated solution = 42.4g
Mass of evaporating dish + dry solid Y = 30.4g
Using the information, determine the solubility of solid Y at 30°C in grams per 100g. (2mks)
Mass of solid Y = 30.4 – 26.2
= 4.2g
Mass of waterin the solution = 42.4 – 30.4g
= 12g
12g of water dissolve 4.2 g of Y
Thus 100g of water dissolves = 100 x 4.2
12
= 35g/100g H2O - A, B, C and D are dyes present in a mixture. C is more soluble than B. A is more soluble than C while D is the least soluble in a given solvent. Draw a round paper chromatogram showing how they would appear when separated using the solvent. (2mks)
- The diagram below shows a section of a model of the structure of element T.
- State the bonding type that exists in element T. (1mk)
- Metallic bond
- In which group of the periodic table does element T belong? Give a reason. (1mk)
- Group 1 elements; has one delocalized electron in each atom.
- State the bonding type that exists in element T. (1mk)
-
- Determine the values of the scalars X and Y in the nuclear equation shown below.
X = 91
Y = 36 - State one application of this type of reaction. (1mk)
- Production of energy
- State one danger associated with exposure of human beings to radioactive substances. (1mk)
- Leads to mutation of genes
- Leads to destruction of the cells of the body
- Determine the values of the scalars X and Y in the nuclear equation shown below.
- The diagram below shows an incomplete set up of the laboratory preparation of dry carbon (IV) oxide. Complete it. (2mks)
- Write down the property of concentrated sulphuric (VI) acid shown in the following reaction. (2mks)
- H2SO4(l)
CuSO4. 5H2O(s)CuSO4(s) +5H2O(l)
- Dehydrating agent
- C(s) + 2H2SO4(I) → CO2(g) + 2H2O(I) + 2SO2(g)
- Oxidizing agent / oxidation
- H2SO4(l)
- When excess chlorine gas is bubbled through dilute sodium hydroxide solution, the resulting solution acts as a bleaching agent.
- Write an equation for the reaction between chlorine gas and sodium hydroxide solution. (1mk)
2NaOH(aq) + Cl2(g) → NaOCl(aq) + NaCl(aq) + H2O(i) - Explain how the resulting solution acts as a bleaching agent. (2mks)
- The resulting mixture contains NaOCl which decomposes to give oxygen which bleaches by adding itself to dye.
- Write an equation for the reaction between chlorine gas and sodium hydroxide solution. (1mk)
- An element P has a relative atomic mass of 88. When a current of 0.5 ampheres was passed through the fused chloride for 32.16 minutes, 0.44g of P were deposited at the cathode. Determine the charge on an ion of P. (1 F = 96500 coulombs) (3mks)
Q = it
= 0.5 x 32.16 x 60
= 964.8C
Numbers of Faradays:
96500C= IF
964.8C = ?
= 964.8
96500
= 0.0099F
0.00999F = 0.44g
? = 88g
= 88 x 0.00999
0.44
= 1.9976F
= 2.0
Charge is 2+ - Matter exists in three states which can be related as shown in the diagram below.
- Name processes P and R. (2mks)
- P sublimation
- R Solidification / freezing
- Explain whether process Q is exothermic or endothermic. (1 ½ mks)
- Exothermic ; slowing down particles release their kinetic energy as the gas condenses into liquid.
- Name processes P and R. (2mks)
- Study the diagram below.
- Give the most likely identity of metal U. (1mk)
- Platinum/ Nichrome/ Copper
- State two observations made in the conical flask. (2mks)
- The hot spiral metal U glows brightly.
- Brown fumes formed / observed above the solution
- Write an equation for the reaction which took place between ammonia and oxygen inside the flask. (1mk)
4NH3(g)+ 5O2(g) → 4NO(g) + 6H2O(l)
- Give the most likely identity of metal U. (1mk)
- In an experiment, soap solution was added to three separate samples of water. The table below shows the volumes of soap solution added in order to form lather with 1000cm3 of each samples before and after boiling.
Sample I Sample II Sample III Volume of soap added to unboiled sample (cm3) 27.0 3.0 10.6 Volume of soap added after boiling the sample (cm3) 27.0 3.0 3.0 - Identify the sample that was likely to be soft water. Explain. (1mk)
- Sample II; it required least amount of soap to lather.
- Explain the change in the volume of soap solution in sample III. (1mk)
- Boiling lead to decomposition of HCO3−hence precipitating Ca2+/Mg2+.
- Give one disadvantage of hard water. (1mk)
- Leads to soap wastage
- Encourages fur forming in boilers and kettles.
- Is uneconomical
- Identify the sample that was likely to be soft water. Explain. (1mk)
- In an experiment to monitor the rate of reaction of magnesium and hydrochloric acid a student recorded the volume of hydrogen produced at regular time intervals and obtained the graph shown below.
- On the same set of axes sketch the curve expected if the experiment is repeated with a few crystals of copper (II) sulphate added to the reactants. (1mk)
- Explain the shape of your curve. (1mk)
- Copper (II) sulphate is a catalyst hence it takes less time for obtaining maximum volume of the hydrogen gas.
- State the factor that can increase the rate of a reaction. (1mk)
- Increase in temperature
- Increase in pressure
- Increase in surface area
- Use of a catalyst
- Light energy
- The grid below is a section of the periodic table. Study it and answer the questions that follow.
- State the name given to the family of B and E. (1mk)
- Alkali metals
- Identify the most reactive metal. (1mk)
- E
- Which type of bond exists in the compound formed by A and F. Explain. (1 ½ mks)
- Ionic/ electrovalent bond; there is complete transfer of electrons from A to F.
- State the name given to the family of B and E. (1mk)
-
- Write the formula of the chief ore (bauxite) from which aluminum is extracted. (1mk)
- Al2O3.2H2O
- Explain the role of molten cryolite in aluminum smelting. (1mk)
- It lowers the melting point of electrolyte from 2015°C to 800°C thus reducing cost of production.
- Write the formula of the chief ore (bauxite) from which aluminum is extracted. (1mk)
- Aluminum does not apparently react with dilute nitric (V) acid to liberate hydrogen gas. Explain. (1mk)
- HNO3 acid is a strong oxidizing agent hence it oxides aluminum on its surface thereby enhancing the protective oxide coating.
- Study the diagram below.
- State the role of manganese (IV) oxide in the set up shown above. (1mk)
- Speeds up/ catalyses the decomposition of hydrogen peroxide to produce oxygen gas.
- State and explain the observation made in tube II. (2mks)
- Solution turns from orange to green; SO2 is a reducing agent hence reduces orange acidified potassium dichromate (VI) to green chromium III ions.
- State the role of manganese (IV) oxide in the set up shown above. (1mk)
- Two manila papers were placed at different levels of a non- luminous flame. Paper A was placed at the lowest part of the flame, while B was placed at the top.
- Indicate the observations made on each manila paper. (1mk)
- Explain the observation made on paper A. (1mk)
- Paper A has not charred/ burned in the middle (as the region consist of colourless zone) where gases are not burning.
- Indicate the observations made on each manila paper. (1mk)
- Starting with 50cm3 of 2.8M sodium hydroxide, describe how a sample of pure sodium sulphate crystals can be prepared. (3mks)
- To 50cm3 of 2.8M sodium hydroxide (NaOH) add 25cm3 of 2.8m sulphuric (vi) acid , (H2SO4).
- Heat the mixture to concentrate.
- Cool it for crystals to form.
- Filter the excess filtrate and dry the residue.
- In an experiment to determine the percentage of magnesium hydroxide in an anti-acid a solution containing 0.5g of the anti-acid was neutralized by 23.0cm3 of 0.10M hydrochloric acid. Given the relative formula mass of magnesium hydroxide is 58. Calculate the:
- Mass of magnesium hydroxide in the anti-acid. (2mks)
Mg(OH)2(aq) + 2HCl(aq) → MgCl2(aq) + H2O(l)
Mole ratio = 1 : 2
No. of moles of the acid = 23 x 0.1 = 0.0023 moles
1000
No. of moles of Mg(OH)2 = 0.0023 = 0.00115 mol
2
Mass of Mg(OH)2 in autacid = 0.00115 x 58
= 0.067g - Percentage of magnesium hydroxide in the anti-acid. (1mk)
Percentage (%) of Mg(OH)2 in anti –acid
0.067 x 100%
0.50
= 13.34%
- Mass of magnesium hydroxide in the anti-acid. (2mks)
- Study the standard electrode potentials in the table below and answer the questions that follow.
EѲvolts
Cu2+(aq) + 2e− → Cu(s) + 0.34
Mg2+(aq) + 2e− → Mg(s) −2.38
Ag+(aq) + e− → Ag(s) +0.80
Ca2+(aq) + 2e− → Ca(s) −2.87- Which of the metals is the strongest reducing agent? (1mk)
- Ca/Calcium reject Ca2+
- What observations will be made if a silver coin is dropped into an aqueous solution of copper (II) sulphate? Explain. (2mks)
- Blue colour of the solution does not charge and the coin does not react. This is because silver is below copper in the reactivity series hence cannot displace copper/ Cu2+ ions from the solution.
- Which of the metals is the strongest reducing agent? (1mk)
- A student used the figure below to investigate the action of dilute sulphuric (VI) acid on some metals. Beaker I and II contained equal volumes of dilute sulphuric (VI) acid. To beaker I, a clean iron rod was dipped and to beaker II, a clean copper rod was dipped.
- Describe the observations made in each beaker.
- Beaker I (1mk)
- There is effervescence producing a colourless gas.
- Beaker II (1mk)
- There is no effervescence since copper does not react with dilute sulphurice (VI) acid.
- Beaker I (1mk)
- Explain observations in (a) above. (1mk)
- Iron is above hydrogen in reactivity series thus displaces hydrogen ions from the acid producing hydrogen gas. Copper on the other hand is below hydrogen in the reactivity series hence cannot displace hydrogen ions from the acid.
- Describe the observations made in each beaker.
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 1 Questions and Answers - Lanjet Mock Exams 2021/2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students