QUESTIONS
- The grid below shows part of the periodic table study it and answer the questions that follow. The letters do not represent the true symbols.
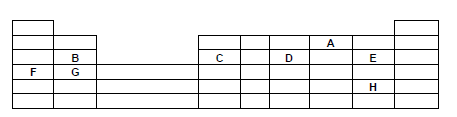
- Which element forms ions with charge of 2-? Explain (2mks)
- What is the nature of the oxide formed by C. (1mk)
- How does the reactivity of H compare with that of E. Explain? (2mks)
- Write down a balanced equation between F and Chlorine. (1mk)
- Explain how the atomic radii of B and C compare. (2mk)
- If the oxides of F and D are separately dissolved in water, state and explain the effects of their aqueous solutions on litmus. (2mks)
- Study the structural formula below and answer the questions that follow.
- CH3 CH2 COOH
- CH3 CH2 CH2OH
-
- Give the systematic name of each compound. (1mk)
- Write the molecular formula of each compound. (1mk)
- How does the boiling point of I compare to that of II? (1mk)
- A gas J is bubbled into concentrated sulphuric (VI) acid. Water is added to the mixture then boiled to yield compound II. Name gas J. (1mk)
- Draw the structural formula of the compound immediately after compound I in the homologous series. (1mk)
- Study the structural formula of the two monomers below and answer the questions that follow.
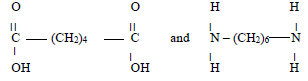
- Name the type of polymerization these monomers would undergo to form a polymer. (1mk)
- Draw the structural formula to represent the polymer formed. (1mk)
- What is the name of the polymer? (1mk)
- State one use of this polymer. (1mk)
- Two cleansing agents are represented below.
Select one that would be suitable for use in water containing magnesium ions. Explain. (2mks)
-
-
- The diagram below is a cross section of a dry cell. Study it and answer the questions that follow
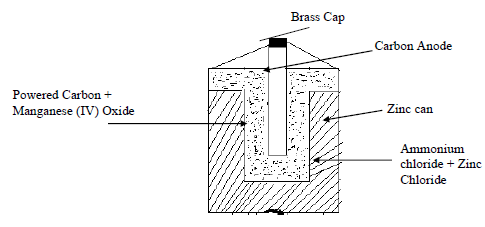
- On the diagram, show with a +ve, the positive terminal. (1mk)
- Write the equation for the reaction at the negative terminal. (1mk)
- A paste of ammonium chloride and zinc chloride is used. What would happen if the mixture becomes dry? Give a reason. (2mks)
- Give one advantage and one disadvantage of dry cells. (1mk)
Brass Cap
Carbon Anode
Zinc can
Ammonium chloride + Zinc Chloride
Powered Carbon + Manganese (IV) Oxide
- Given the following standard electrode potentials.
Mg2+ (aq) + 2e- Mg(s) -2.37v
Ce 4+ (aq) + e Ce3+ (aq) +1.61v- Write the cell representation for a voltaic cell from the above half equations. (1mk)
- Calculate the voltage of the cell in. (i) above (1mk)
- Write the overall electrochemical equation for the cell reaction. (1mk)
- The diagram below shows the apparatus that can be used to electroylse acidified water to obtain gas X and gas Y.
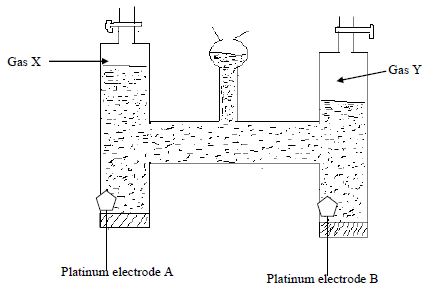
- Identify the electrode where oxidation takes places. (1mk)
- Explain why hydrochloric acid may not be used to acidify the water. (1mk)
- Write the half equation for the reaction at the anode. (1mk)
- During electrolysis, a current of 3 amperes is passed through the solution for 45 minutes and 30 seconds. What volume of gas will be liberated at the anode? (Molar gas volume = 24.0dm3, 96,500C) (3mks)
- The diagram below is a cross section of a dry cell. Study it and answer the questions that follow
-
- The set up below was used to prepare hydrogen gas.
- Complete the diagram to show how a dry sample of the gas can be collected. (3mks)
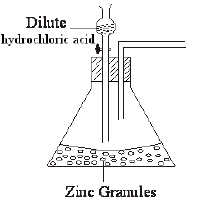
- Write an equation for the reaction producing hydrogen gas. (1mark)
- How can the rate of production of the gas be increased in the above set up? (1mark)
- Complete the diagram to show how a dry sample of the gas can be collected. (3mks)
- Dry hydrogen gas was passed over heated copper (II) oxide in a combustion tube as shown below.
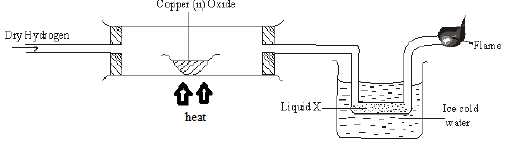
- State and explain the observation made in the combustion tube. (2marks)
- Write an equation for the reaction that took place in the combustion tube. (1mk)
- Describe one chemical test than can be used to prove the identity of liquid X. (2marks)
-
- When magnesium oxide is used in place of copper (II) oxide, no liquid is formed in the U-tube dipped in ice cold water. Explain. (1mark)
- Write an equation for the reaction at the flame point. (1mark)
- The set up below was used to prepare hydrogen gas.
- Below is a flow diagram for the manufacture of sulphuric (VI) acid. Study it and answer the questions that follow.
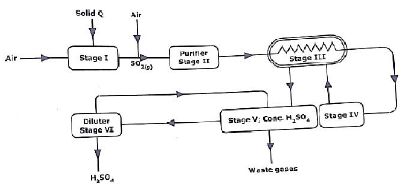
- Name this process. (1mk)
- Identify solid Q (1mk)
- Write equations for reactions in (3mks)
Stage I
Stage IV
Stage V - Name one impurity removed in stage II. (1mk)
- Name the catalyst that is less preferred for this method. Give a reason. (2mks)
- State two functions of the heat exchanger. (1mk)
- How is pollution effect remedied in this process? (1mk)
- The concentration of the sulphuric(VI) acid can be checked by titration. A sample of the sulphuric(VI) acid was analysed as follows.
100cm3 of the acid was diluted with water to make 1.0dm3 of solution.The diluted sulphuric (VI) acid was then titrated with aqueous sodium hydroxide .25cm3 of 0.1M aqueous sodium hydroxide required 20.0cm3 of the diluted sulphuric (VI) acid for the neutrilisation. Calculte the concentration of the original sulphuric (VI) acid sent for analysis. (3marks)
- The flow chart below represents the steps and processes involved in the extraction of copper from copper pyrites. Study the flow chart and answer the questions that follow.
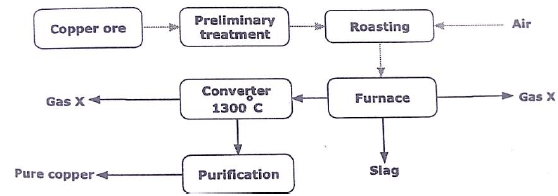
- Write the formula of another ore from which copper can be extracted from. (1mk)
- Name the method used during the preliminary treatment of the ore? (1mk)
- Name
- Gas X. (1mk)
- The slag. (1mk)
- Write an equation that occurs in (2mks)
- Furnace
- converter
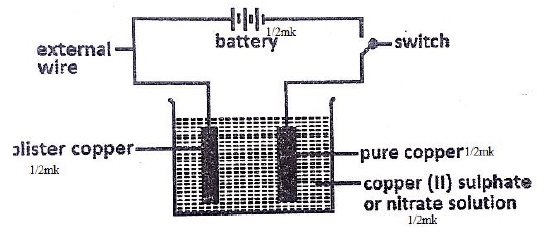
- Draw a set up to show how purification of copper takes place. (2mks)
- State one environmental effect associated with extraction of copper metal. (1mk)
- State two uses of copper. (1mk)
-
- A salt K has the following properties:
- Dissolves in water to form a colourless solution
- Its solution gives no visible reaction with barium chloride
- Its solution forms white precipitate with lead (II) nitrate, sodium hydroxide and ammonia solutions
- The precipitates formed with the alkalis are soluble in excess.
Identify solid K. (1mk)
-
- At 25°C, 50g of substance X were added to 100g of water to make a saturated solution.
What is meant by a saturated solution? (lmk) - The table below gives the solubility of substance X at different temperatures
Temperature ºC 14 24 33 40 46 52 Solubility g/ 100g H2O 24 36 50 62 72 90 - Plot a graph of the solubility of substance X (vertical axis) against temperature (3mks)
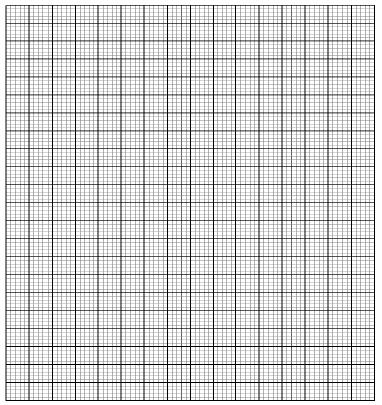
- Using the graph determine the solubility of substance X at 20°C (1mk)
- Determine the mass of substance X that remained undissolved given that 90g of substance X were added to 100cm3 of water and warmed to 35°C. (2mk)
- Calculate the molarity of the solution at 30°C. (Relative formula mass of X =122.5). (2mks)
- Plot a graph of the solubility of substance X (vertical axis) against temperature (3mks)
- At 25°C, 50g of substance X were added to 100g of water to make a saturated solution.
- A salt K has the following properties:

MARKING SCHEME
- The grid below shows part of the periodic table study it and answer the questions that follow. The letters do not represent the true symbols.

- Which element forms ions with charge of 2-? Explain (2mks)
A(1)- gains two electrons to become stable(1) - What is the nature of the oxide formed by C. (1mk)
amphoteric - How does the reactivity of H compare with that of E. Explain? (2mks)
H is less reactive than E(1)
H has a larger atomic radius hence lower attraction to electrons(1) - Write down a balanced equation between F and Chlorine. (1mk)
2F(s) + Cl2(g) 2FCl(s)
2Na(s) + Cl2(g) 2NaCl(s) - Explain how the atomic radii of B and C compare. (2mk)
C has a smaller atomicradius than B(1), Chas more protons hence stronger nuclear charge/attraction for outermost electrons(1) - If the oxides of F and D are separately dissolved in water, state and explain the effects of their aqueous solutions on litmus. (2mks)
Oxideof F changes litmus to blue (½)–dissolves in water to form alkaline/ basic solution(½)
Oxide of D changes litmus to red(½)- dissolves in water to form acidic solution(½)
- Which element forms ions with charge of 2-? Explain (2mks)
- Study the structural formula below and answer the questions that follow.
- CH3 CH2 COOH
- CH3 CH2 CH2OH
-
- Give the systematic name of each compound. (1mk)
- propanoic acid(½)
- propan-1-ol ( ½ )
- Write the molecular formula of each compound. (1mk)
- C3H6O2 ½
- C3H8O ½
- How does the boiling point of I compare to that of II? (1mk)
I has a higher boiling point than II. (½)
I has more hydrogen bonds than II(½) - A gas J is bubbled into concentrated sulphuric (VI) acid. Water is added to the mixture then boiled to yield compound II. Name gas J. (1mk)
propene - Draw the structural formula of the compound immediately after compound I in the homologous series. (1mk)
Draw an open structural formula of a positional isomer of butene
- Give the systematic name of each compound. (1mk)
- Study the structural formula of the two monomers below and answer the questions that follow.
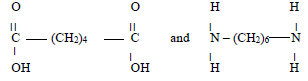
- Name the type of polymerization these monomers would undergo to form a polymer. (1mk)
condensation - Draw the structural formula to represent the polymer formed. (1mk)
- What is the name of the polymer? (1mk)
Nylon - State one use of this polymer. (1mk)
Substitute for cotton in the textile industry, ropes carpets, brushes, fishing nets, parachutes
- Name the type of polymerization these monomers would undergo to form a polymer. (1mk)
- Two cleansing agents are represented below.

Select one that would be suitable for use in water containing magnesium ions. Explain. (2mks)
Detergent R(1)
Does not form scum(1)
-
-
- The diagram below is a cross section of a dry cell. Study it and answer the questions that follow

- On the diagram, show with a +ve, the positive terminal. (1mk)
Right at the top and in contact with the carbon rod// at the brass cup - Write the equation for the reaction at the negative terminal. (1mk)
Zn(s) → Zn2+(aq) + 2e- - A paste of ammonium chloride and zinc chloride is used. What would happen if the mixture becomes dry? Give a reason. (2mks)
The cell would not produce any current// cell stop working//no reaction (1)
Ions are not mobile in solid state (1) - Give one advantage and one disadvantage of dry cells. (1mk)
Advantage – portable//cheap//convenient to use(½)
Disadvantage – not rechargeable//cannot produce continuous supply of electric current// cause environmental pollution(½)
- On the diagram, show with a +ve, the positive terminal. (1mk)
- Given the following standard electrode potentials.
Mg2+ (aq) + 2e- → Mg(s) -2.37v
Ce 4+ (aq) + e → Ce3+ (aq)+1.61v- Write the cell representation for a voltaic cell from the above half equations. (1mk)
Mg(s)/Mg2+(aq)//Ce4+(aq)/ Ce3+(aq) - Calculate the voltage of the cell in. (i) above (1mk)
+1.61 – (- 2.37)
=+3.98V - Write the overall electrochemical equation for the cell reaction. (1mk)
Mg(s) +2Ce4+(aq) → Mg2+(aq) + 2Ce3+(aq)
- Write the cell representation for a voltaic cell from the above half equations. (1mk)
- The diagram below shows the apparatus that can be used to electrolyse acidified water to obtain gas X and gas Y.

- Identify the electrode where oxidation takes places. (1mk)
A - Explain why hydrochloric acid may not be used to acidify the water. (1mk)
Oxidation potential of chloride ions is very high, thus chances of chloride ions being oxidized to liberate chlorine gas at the expense of hydroxide ions which should give oxygen upon oxidation - Write the half equation for the reaction at the anode. (1mk)
4OH-(aq) → 2 H2O(I) + O2(g) + 4e- - During electrolysis, a current of 3 amperes is passed through the solution for 45 minutes and 30 seconds. What volume of gas will be liberated at the anode? (Molar gas volume = 24.0dm3, 96,500C) (3mks)
T= 45 x 60 + 30 = 2730sec
Q= IT
= 3 x 2730
= 8190C(½)
96500 x 4= 386000C(1)
386000C 24000cm3
8190C 8190 x 24000(1)
386000
=509.22cm3(½)
- Identify the electrode where oxidation takes places. (1mk)
- The diagram below is a cross section of a dry cell. Study it and answer the questions that follow
-
- The set up below was used to prepare hydrogen gas.
- Complete the diagram to show how a dry sample of the gas can be collected. (3mks)

Drying agent(1) -conc. H2SO4//Anhy. CaCl2// CaO
Method of collection(1)- upward delivery// useof syringe
Workability(1) - Write an equation for the reaction producing hydrogen gas. (1mark)
Zn(s) + 2HCl(aq) → ZnCl2(aq) H2(g) - How can the rate of production of the gas be increased in the above set up? (1mark)
adding copper(II) sulphate II use of zinc powder
- Complete the diagram to show how a dry sample of the gas can be collected. (3mks)
- Dry hydrogen gas was passed over heated copper (II) oxide in a combustion tube as shown below.

- State and explain the observation made in the combustion tube. (2marks)
Black copper(II) oxide changes to brown(1)
Copper (II) oxide is reduced to copper by hydrogen gas(1) - Write an equation for the reaction that took place in the combustion tube. (1mk)
CuO(s) + H2(g) Cu(s) + H2O(l)
- State and explain the observation made in the combustion tube. (2marks)
- Describe one chemical test than can be used to prove the identity of liquid X. (2marks)
Mix with anhydrous/white copper(II) sulphate (1).Changes to blue(1)//
Mix with anhydrous/blue cobalt chloride (1).Changes to pink(1) -
- When magnesium oxide is used in place of copper (II) oxide, no liquid is formed in the U-tube dipped in ice cold water. Explain. (1mark)
Magnesium ismore reactive than hydrogen hence cannot be reduced by hydrogen - Write an equation for the reaction at the flame point. (1mark)
2H2(g) +O2(g) → 2H2O(l)
- When magnesium oxide is used in place of copper (II) oxide, no liquid is formed in the U-tube dipped in ice cold water. Explain. (1mark)
- The set up below was used to prepare hydrogen gas.
- Below is a flow diagram for the manufacture of sulphuric (VI) acid. Study it and answer the questions that follow.

- Name this process. (1mk)
contact - Identify solid Q (1mk)
Sulphur//iron(II) sulphide// zinc sulphide // copper pyrites // lead II sulphide
Accept formulae - Write equations for reactions in (3mks)
Stage I S(s) + O2(g) → SO2(g)
Stage IV 2SO2(g) +O2(g) → 2SO3(g)
Stage V SO3(l) +H2SO4(l) → H2S2O7(l) - Name one impurity removed in stage II. (1mk)
Dust particles - Name the catalyst that is less preferred for this method. Give a reason. (2mks)
Platinum(1)
Expensive// easily poisoned (1mk) - State two functions of the heat exchanger. (1mk)
Preheat incoming mixture(½)
Cools hot mixture from catalytic chamber(½) - How is pollution effect remedied in this process? (1mk)
Through scrubbing// SO2 is absorbed in a chimney lined with calcium hydroxide - The concentration of the sulphuric(VI) acid can be checked by titration. A sample of the sulphuric(VI) acid was analysed as follows.
100cm3 of the acid was diluted with water to make 1.0dm3 of solution.The diluted sulphuric (VI) acid was then titrated with aqueous sodium hydroxide .25cm3 of 0.1M aqueous sodium hydroxide required 20.0cm3 of the diluted sulphuric (VI) acid for the neutrilisation. Calculte the concentration of the original sulphuric (VI) acid sent for analysis. (3marks)
Moles of NaOH = 25 x 0.1
1000
= 0.0025moles 1/2
2NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + 2H2O(l)// MR 2:1 1/2
Moles of H2SO4 = 0.0025/2 = 0.00125moles 1/2
Moles in 1dm3 = 1000 x 0.00125 1/2
20
= 0.0625moles 1/2
100M1 = 0.0625 x 1000
M1=0.625M 1/2
- Name this process. (1mk)
- The flow chart below represents the steps and processes involved in the extraction of copper from copper pyrites. Study the flow chart and answer the questions that follow.

- Write the formula of another ore from which copper can be extracted from. (1mk)
Cu2O// Cu2S//CuCO3.Cu(OH)2 - Name the method used during the preliminary treatment of the ore? (1mk)
Froth flotation - Name
- Gas X. sulphur(IV) oxide (1mk)
- The slag. (1mk)
Iron (II) silicate
- Write an equation that occurs in (2mks)
- Furnace
2CuFeS2(s) + 4O2(g) Cu2O(S) + 2FeO(s) +3SO2(g) - converter
Cu2S(l) +2 Cu2O(l) 6Cu(l) + SO2(g)
- Furnace
- Draw a set up to show how purification of copper takes place. (2mks)
- State one environmental effect associated with extraction of copper metal. (1mk)
SO2 forms acid rain which corrodes stoneworks// any one
Deformation of land// formation of gullies - State two uses of copper. (1mk)
Making electrical wires and contacts in switches, plugs and sockets(½)
Making soldering instruments(½)
Making alloys –brass/bronze/German silver
Making coins and ornaments any 2
- Write the formula of another ore from which copper can be extracted from. (1mk)
-
- A salt K has the following properties:
- Dissolves in water to form a colourless solution
- Its solution gives no visible reaction with barium chloride
- Its solution forms white precipitate with lead (II) nitrate, sodium hydroxide and ammonia solutions
- The precipitates formed with the alkalis are soluble in excess.
Identify solid K. (1mk)
ZnCl2// zinc chloride
-
- At 25°C 50g of substance X were added to 100g of water to make a saturated solution.
What is meant by a saturated solution? (lmk)
A solution which contains as much solute as can dissolve at a particular temperature in the presence of undissolved solid. - The table below gives the solubility of substance X at different temperatures
Temperature ºC 14 24 33 40 46 52 Solubility g/ 100g H2O 24 36 50 62 72 90 - Plot a graph of the solubility of substance X (vertical axis) against temperature (3mks)
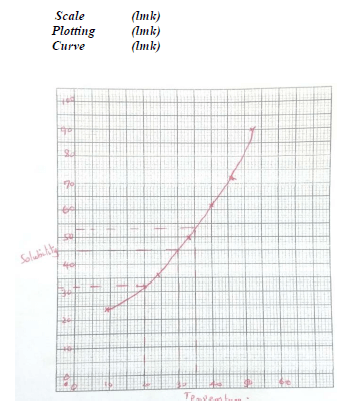 Scale (lmk)
Scale (lmk)
Plotting (lmk)
Curve (lmk) - Using the graph
I determine the solubility of substance X at 20ºC (1mk)
32g /100g of water ±2
(Read from candidate graph) - Determine the mass of substance X that remained undissolved given that 90g of
substance X were added to 100cm3 of water and warmed to 35°C. (2mk)
Solubility at 35°C 53g/l00g of H2O±2
Mass dissolved = 53g
Mass un dissolved =90- 53 = 47g ±2 - Calculate the molarity of the solution at 30°C. (Relative formula mass of
X =122.5). (2mks)
Solubility of X at 30°C = 44g/l00g ±2 H2O
Mole of X = 44 0.3592mo1
122.5
0.3592mol contained in 100cm3
Y mol contained in 1000cm3
Y =0.3592 x 1000 =3.592M
100
- Plot a graph of the solubility of substance X (vertical axis) against temperature (3mks)
- At 25°C 50g of substance X were added to 100g of water to make a saturated solution.
- A salt K has the following properties:
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 2 Questions and Answers - Butere Mock Exams 2021.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students