Instructions
- Write your named and index number in the spaces provided above
- Answer all questions in the spaces provided in the question paper
- Mathematical tables (KNEC) and silent electronic calculators may be used
- All workings must be clearly shown where necessary
- Candidates should answer the questions in English
For examiners use only
|
Question |
Maximum score |
Candidate’s score |
|
1 |
12 |
|
|
2 |
10 |
|
|
3 |
12 |
|
|
4 |
12 |
|
|
5 |
12 |
|
|
6 |
12 |
|
|
7 |
10 |
QUESTIONS
- The grid below shows part of the periodic table Use it to answer question the follow The letters do not represent actual symbols
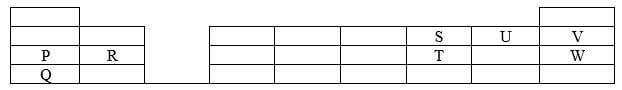
- Which of the elements has the highest atomic radius? Explain (2 marks)
- Identify the most reactive non-metal Explain (2 marks)
- Give the electron configuration of:
- Element S (1/2 mark)
- Element Q (1/2 mark)
- Compare the atomic radius of P and R Explain (2 marks)
- Given that the atomic mass of W is 40 Write down the composition of its nucleus (1 mark)
- Write the formula of compounds formed between
- Element P and S (1 mark)
- Element R and T (1 mark)
- Give the formula of one stable Ion with an electron arrangement of 28 which is
- Negatively charged (1 mark)
- Positively charged (1 mark)
- Study the flow chart below and answer the question that follows
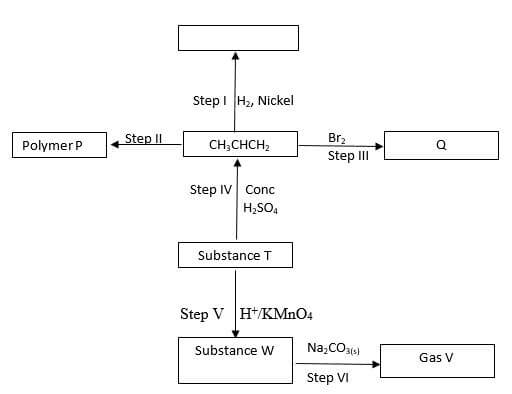
- Identify the following
- Substance W …………………………………………………………… (1mark)
- Gas V ………………………………………………………………… (1 mark)
- Name the processes involved in the following steps
- Step I …………………………………………………………………… (1 mark)
- Step II ………………………………………………………………… (1 mark)
-
- What type of reaction is taking place in step V ( 1mark)
- Draw the structure and give their IUPAC name for the following compounds (4 marks)
Compound
Structure
Name
Q
(1 mark)
( 1mark)
P
(1 mark)
(1 mark)
- Write the equation that took place in step III (1 mark)
- Identify the following
- Study the standard electrode potentials for the half cells given below and answer the questions that follow
Eo (volts)
A+(aq) + e- → A(s) → -292
B+(aq) + e- → B(s) + 052
C+(aq) + e- → 1/2C2(g) 000
D2+ + 2e- → D(s) -044
1/2E2(aq) + e- → E(aq) +136- Identify the strongest oxidising agent Explain (2 marks)
-
- Which two half cells would produce the highest potential difference combined (1 mark)
- Give the cell diagram for b (i) above (1mark)
-
- Explain whether the reaction represented by the equation below can take place (2 marks)
2A+(aq) + D(s) → A(s) + D2+(aq) - 90cm3 of acidified water was electrolysed using the set up below
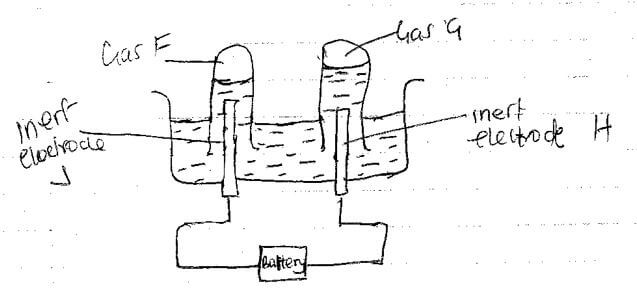
- Identify electrodes H and J
H - …………………………………………………………………… (1/2 mark)
J - …………………………………………………………………… (1/2 mark) - Describe how gas F can be identified (2marks)
- In the above experiment 5A of electricity was passed through the acidified water for 3 minutes and 21 seconds Calculate the volume of gas G produced at room temperature and pressure molar gas volume at rtp= 24000cm3/ F=96 500c (3 marks)
- Identify electrodes H and J
- Explain whether the reaction represented by the equation below can take place (2 marks)
-
- The following results were obtained in an experiment
Mass of crucible + Lid = 1952g
Mass of crucible + Lid + Magnesium ribbon = 2036g
Mass of crucible + Lid + Magnesium oxide = 2092g- Use the results to determine the percentage mass of magnesium and oxygen in magnesium oxide (2 marks)
- Determine the empirical formula of magnesium oxide
(Mg = 24, O = 16)
- Sodium hydroxide pellets were accidentally mixed with sodium chloride 88g of the mixture were dissolved in water to make one litre of solution 50cm3 of the solution was neutralised by 20cm3 of 025M Sulphuric acid
- Write an equation for the reaction that took place (1mark)
- Calculate the:
- number of moles of the substance that reacted with sulphuric acid (2 marks)
- number of moles of the substances that would react with sulphuric acid in the one litre solution (2 marks)
- The percentage of sodium chloride in the mixture (2 marks)
(H = 10, Na = 230, Cl = 355, O = 160)
- The following results were obtained in an experiment
-
- In an experiment to determine the heat of combustion of ethanol the following data was collected
Volume of water = 450cm3
Initial temperatures of water = 25ºc
Final temperature of water = 465ºc
Mass of ethanol + lamp before heating = 1255g
Mass of ethanol + lamp after heating = 1240gCalculate:
- Heat evolved during the experiment (Density of water = 1g/cm3, specific heat capacity of water
= 42k/kg-1k-1 (2 marks) - Molar heat of combustion of ethanol (2 marks)
(C = 12, O = 16, H = 1)
- Heat evolved during the experiment (Density of water = 1g/cm3, specific heat capacity of water
- Write the equation for the complete combustion of ethanol (1 mark)
- The molar heat of combustion obtained from an experiment like the one above is usually lower than the theoretical value
Explain (2 marks) - The molar heat of combustion of hydrogen is given as -286K/mol-2
- Write the thermochemical equation for the reaction (1 mark)
- Draw an energy level diagram for the reaction in b (i) above (2 marks)
-
- What is a fuel? (1 mark)
- State two factors considered when choosing fuel (1 mark)
- In an experiment to determine the heat of combustion of ethanol the following data was collected
- The factors which affects the rate of reaction between lead (II) carbonate and dilute nitric (V) acid were investigated by carrying out three experiments
Experiment number
Lead (II) carbonate
Concentration of nitric (V) acid
1
Lumps
4M
2
Powdered
4M
3
Lumps
2M
- Other than concentration, name another factor that was investigated in the experiment (1mark)
- For each experiment the same volume of acid (excess) and mass of lead (II) carbonate were used and the volume of gas liberated measured with time
- Draw set up that can be used to investigate the rate of reaction for one of the experiments (3 marks)
- On the grid provided, sketch the curves obtained when the volume of gas produced was plotted against time for each of the experiments and label each as 1,2 or 3 (3 marks)
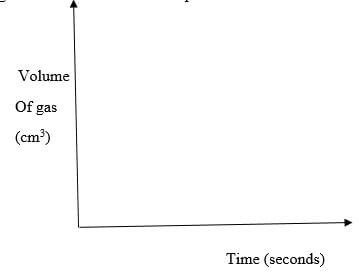
- Write an equation for the reaction that took place (1mark)
- If the experiments were carried out using dilute hydrochloric acid instead of dilute nitric (V) acid, the reaction would start, slow down and eventually stop Explain (2 marks)
- Bromine gas dissolves in water according to the following equation
Br2(g) + H2O(l) ⇌ 2H+(aq) + Br-(aq) + OBR-(aq)
Yellow/orange Colourless - State and explain the observation made when hydrochloric acid is added to the mixture at equilibrium (2 marks)
- In an experiment to determine the solubility of potassium chlorate, the following results were obtained
Total volume of water added (cm3)
10.0
20.0
30.0
40.0
50.0
Mass of potassium chlorate
5.0
5.0
5.0
5.0
5.0
Temperature at which crystals appear (oc)
80.0
65.0
55.0
45.0
30.0
Solubility of potassium chlorate (g/long H2O)
- Complete the table to show the solubility of potassium chlorate at different temperatures (3 marks)
- Plot a graph of mass of potassium chlorate per 100g water against temperature at which crystals from (3 marks)
- From the graph, determine:
- the solubility of potassium chlorate at 40ºC (1 mark)
- The temperature at which the solubility of potassium chlorate is 35g/100g water (1 mark)
- Explain the shape of the graph (1 mark)
- State one application of solubility and solubility curves (1 mark)
MARKING SCHEME
-
- Q√ 1- has highest number of occupied energy levels √
- U√1- most electro negative/highest to attract tendency the highest elector affinity√
-
- S – 2.8 √1/2
- Q – 2,8,8,1 √1/2
- P is larger than R √1 nuclear charge increase across the period //R has more protons than P. √1
- Atomic mass = P + n
18 + n = 40
N = 40 – 18
N = 22
Composition P = 18√1/2 , n = 22√1/2 -
- P2S√1/2 //Na2O
- RT/MgS√1
-
- U- or S2- // F- or O2-
- P+ or R2+√1 // Na+ or Mg 2+
-
-
- W – propanoic acid /CH3CH2COOH√1
- Gas V – carbon (iv). Oxide √1 / CO2
-
- Hydrogenation √1
- Polymerisation √12
-
- Oxidation √1
- 1,2 dibromopropane√1

- CH3CHCH2(g) + Br2(g) → CH3CHBrCH2Br(g) √1
-
-
-
- E√1Itt has the more positive√1 standardelectrode potential
-
- A and E half cells√1
- A(s)/A+(aq) // ½ E2(s)/E-(aq) Pt EƟ=+4.28V√1/2
- emf = Ered – Eoxid
=2.92 - -(-0.44) √1
= -2.48V
Overall emf is negative, the reaction√1 does not take place Orz√1
2A+ + 2e- →A(s) EƟ – 2.92
D(s) →D2+ + 2e- EƟ + 0.44
2A+(aq) + D(s) →A(s) + D2+(aq) E – 2.48
-
- H -Anode √1/2
J – Cathode √1/2 - a burning splint is introduced at the mouth a test tube containing gas F√1
A dep sound is produced √1
Accept: It goes off/extinguishes with a loop ‘sound
Reject – It burn with a ‘pop’ sound - Q = it Q = 5x [(3x60) + 21]
= 5 x 201
= 1005c√14OH(aq) →2H2O(l) + O2(g) + 4e- √1
If = 96500c
4 x 96500c ⟶24000cm3
1005 c→ ?
1005c x 24000 √1/2
4 x 96500c = 62.4870466
= 62.48cm3√1/2
- H -Anode √1/2
-
-
-
- Mass of magnesium oxide = 20.92 – 19.52
=1.40g - Mass of magnesium = 20.36 = 19.52
= 0.84g√1/2
Mass of oxygen = 20.92 – 20.36
= 0.56g√1/2
Percentage mass of oxygen in magnesium oxide = (0.56 )/1.40 x 100%
= 40%√1/2
- Mass of magnesium oxide = 20.92 – 19.52
-
The empirical formula is MgO√1Element
Mg
O
% composition by mass
R.A.M
No. of moles
Mole ration
60
24
= 60 = 2.5 2
24
=2.5
2.51 1/2
40
16
= 40 2.5 1/2
16
=2.5
2.51 1/2
- 2HaOH(aq) + H2SO4(aq) →Na2SO4(aq) + 2H2O(l) √1/2
- Moles of sulphuric acid that reacted with the solution = (20 x 0.25)/1000 √1/2
= 0.005 moles √1/2
NaOH : H2SO4
2:1 √1/2
∴Moles of sodium hydroxide → 0.005 x2
0.01 moles √1/2 - If 50cm3 contains 0.01 moles
∴1000cm3 contains = (1000 x 0,01 )/50√1/2
0.2 moles√1 - RMM of NaOH = 23 + 16 + 1
= 40
Mass of NaOH in 1 litre ⇒0.2 x 40
= 8g√1/2
Mass of sodium chloride in the mixture = (8.8 – 8)g
= 0.8g√1/2
Percentage mass of sodium chloride = 0.8/8.8 x 100%√1/2
= 9.09%√1/2
- Moles of sulphuric acid that reacted with the solution = (20 x 0.25)/1000 √1/2
-
-
-
- Change in temperature ∆T = 46.5 – 25
= 21.5k√1/2
Heat evolved ∆H =MC∆T
= 0.45 x 4.2 x 21.5√1/2
= 40.635KJ√1 - Mass of ethanol burned = 125.5 – 124.0
= 1.5g
Molar mass of C2H5OH = (2 x 12) + (6 x 1) + 16
= 46√1/2
No.of moles = 1.5/46 √1/2
= 0.032608695652173 moles
Molar heart of combustion of ethanol =(40.635 )/( 0.032608695652173)√1/2
= -1,246.14Kjmol-1 √1/2
- Change in temperature ∆T = 46.5 – 25
- CH3H2OH(l) + 3O2(g) →2CO2(g) + 3H2O(l) √1
- Heat last to the surrounding √1 and that absorbed by apparatus√1 is not accounted for.
-
- H2 (g) + ½O2(g) →H2O(g) ∆H= -286kJmol-1√1/2
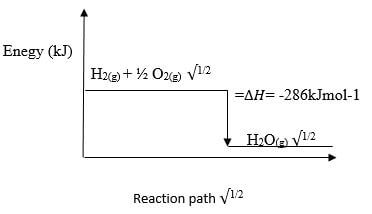
Reaction path √1/2
- H2 (g) + ½O2(g) →H2O(g) ∆H= -286kJmol-1√1/2
-
- A fuel is a substance that produces useful energy when it undergoes a chemical or nuclear reaction √1
-
- Cost
- Heating value
- Availability
- Environmental effects
- Ease of storage
- Ease of transportation
- Ease and rate of combustion
(Any two for mk)
-
-
- surface area √1 / size of particles
-
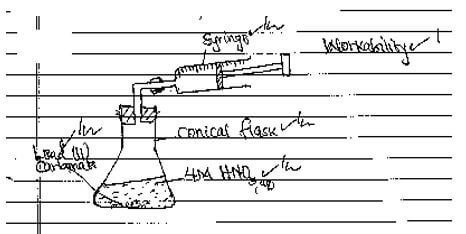
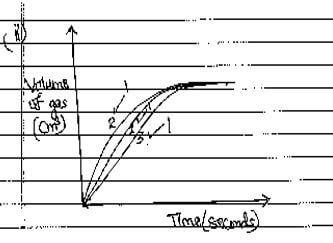
- PbCO3(s) + 2HNO3(aq) →Pb(NO3)2(aq) + CO2(g) + H2O(l)√1
(Penalize ½ mk for missing/wrong state symbols)
- Insoluble lead (II) chloride formed coat the surface of lead (II) carbonate. √1 This prevents further reaction √1
- Colourless solution changes to yellow/orange√1. Addition of hydrochloric acid increases the concentration of H+ ions and the equilibrium shifts from right to left √1
-
NB√1mk for expressing solubility values to the same number of decimal places.Solubility of potassium chlorate
(g/100gH2O
50.0 1
25.0 1
16.7 1
12.5 1
10.0 1
- S-1/2
L- ½
P – 1
C – 1
3 -
- 11g/100gH2O√1
- 72ºc (±0.5) √1
- solubility of potassium, chlorate increases with increase in temperature √1/more potassium chlorates dissolves as temperature rises√1
- Extraction of sodium carbonate from Trona
Extraction of sodium chloride
(Any one)
Download Chemistry Paper 2 Questions and Answers - Arise and Shine Mock Exams 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students
