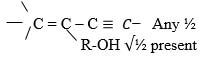Instructions to candidates.
- Write your name and index number in the spaces provided above.
- Answer all questions in the spaces provided in the question paper.
- Mathematical tables (KNEC) and silent electronic calculators may be used.
- All workings must be clearly shown where necessary.
- Candidates should answer the questions in English.
For examiners use only.
|
Question |
Maximum score |
Candidate’s score |
|
1 |
22 |
|
|
2 |
10 |
|
|
3 |
8 |
|
|
Total score |
40 |
QUESTIONS
- You are provided with
- 0.3g of metal F.
- 100cm3 of 1.0M hydrochloric acid solution labelled as solution G.
- 120cm3 of 0.1M sodium hydroxide solution, labelled as solution H.
- Screened methyl orange indicators solution.
- You are required to determine the Relative Atomic Mass of metal F.
Procedure- Using a burette, measure 50.0cm3 of solution G into a clean 250ml beaker.
- Add the WHOLE AMOUNT of F provided into the beaker containing 50.0cm3 of solution G and stir well with a glass rod until ALL the solid metal reacts completely.
- Transfer the mixture left in the beaker after the reaction into a 250ml Volumetric flask. Rinse the beaker as well as the glass rod with distilled water and transfer ALL the rinsings into the volumetric flask. Make up the volume of the solution in the volumetric flask up to the calibration mark with distilled water, cover the flask with a stopper, shake well and label as solution Q.
- Fill a clean burette with solution Q.
- Pipette 25.0cm3 of solution H into a 250ml conical flask, add 3 drops of screened methyl orange indicator solution and titrate against solution Q from the burette.
A change in colour of the mixture from green to pink marks the end point of titration.
Record your results in table 1. - Repeat the titration TWO more times to complete table I.
Table I
(4 marks)Titration
1
2
3
Final burette reading, cm3
Initial burette reading,cm3
Volume of solution Q used,cm3
Average volume of Q used, cm3 ………………………………………………….. (1 mark) - Calculate:
- Calculate the number of moles of HCl in 50.0cm3 of solution G. (1 mark)
- Determine the number of moles of NaOH in 25.0cm3 of solution H. (1 mark)
- Determine the number of moles of HCl in the average volume of solution Q useD in the titration. (1 mark)
- Calculate the moles of HCl left unreacted after the reaction between F and solution G. (1 mark)
- Determine the moles of HCl that reacted with metal F. (1 mark)
- Given that metal F forms a divalent cation, determine the moles of metal F that reacted with hydrochloric acid. (1 mark)
- Determine the Relative Atomic mass of metal F. (1 mark)
- You are provided with
- 2.00g of solid K.
- A thermometer
- Distilled water
- Boiling tube
- Hot water bath.
You are required to determine the temperatures at which solutions of known concentrations of compound K becomes saturated and plot solubility curve.
Procedure.- Transfer the whole amount of solid K supplied to you into clean dry boiling tube.
- Using a burette, add 5.0cm3 of distilled water into the boiling tube with solid K
- Put the boiling tubeinto a beaker of hot water bath and warm the boiling tube, while continuously stirring the content with thermometer, until the crystals of K dissolve/disappear
(DO NOT BREAK THE THERMOMETER) - Remove the boiling tube from the hot water bath and allow the content to cool slowly while stirring with the thermometer. Not the temperature at which crystals
FIRST form/reappear and record this temperature in Table 2. - Add a further 2.00cm3 of distilled water from the burette into the boiling tube containing the mixture and repeat steps (c) and (d) above. Continue this way until the volume of water added to boiling tube is 5.00cm3.
- Complete Table 2 by calculating the solubility of compound K in water at different temperatures.
(6 marks)Total volume of
water added (cm3)
Temperature at which
crystals first appear (oc)
Solubility of compound K
in water (g/100g water)
5.00
7.00
9.00
11.00
13.00
15.00
- On the grid provided plot a graph of solubility of compound K (vertical axis) against temperature. (3 marks)
- From your graph determines the solubility of K in water at 25.0ºc. (1 mark)
- You are required to determine the Relative Atomic Mass of metal F.
- You are provided with 10cm3 of solution R containing TWO cations and ONE anions carry out the tests below and record your observations and inferences in the spaces provided.
- Add 20cm3 of 2M sodium hydroxide to all of solution R provided. Shake well. Filter the mixture into a conical flask. Retain both the filtrate and residue.
Observation
Inference
(1/2 mark)
(1/2 mark)
- To about 2cm3 of the filtrate, add 1cm3 of 2cm3 of 2M nitric acid. Retain the mixture.
Divide the mixture in (b) above into TWO portionsObservation
Inference
(1/2 mark)
(1/2 mark)
- To the FIRST portion, add aqueous sodium hydroxide solution drop wise until in excess.
Observation
Inference
(1 mark)
(1 mark)
- To the SECOND portion, add 2M aqueous ammonia solution DROPWISE until in excess.
Observation
Inference
(1 mark)
(1 mark)
- To the FIRST portion, add aqueous sodium hydroxide solution drop wise until in excess.
- To about 2cm3 of the filtrate, add 3 drops of 2M hydrochloric acid
Observation
Inference
(1/2 mark)
(1/2 mark)
- To about 2cm3 of the filtrate, add about 1cm3 of acidified Barium chloride solution
Observation
Inference
(1/2 mark)
(1/2 mark)
- To the RESIDUE add about 5cm3 of dilute nitric acid and filter into a clean test tube. To about 2cm3 of this filtrate add 2M aqueous. Ammonia solution dropwise until in excess and filter into clean test tube.
Observation
Inference
(1 mark)
(1 mark)
- Add 20cm3 of 2M sodium hydroxide to all of solution R provided. Shake well. Filter the mixture into a conical flask. Retain both the filtrate and residue.
- You are provided with solid Z.
Carry out tests below. Write your observations and inferences in the spaces provided.- Scoop a little of solid Z (using a clean spatula and burn it in a |Bunsen burner flame.
Observation
Inference
(1 mark)
(1 mark)
- To the remaining portion, add about 6m3 of distilled water and shake. Divide the mixture into two portions
Observation
Inference
(1 mark)
(1 mark)
- To the second portion, add the whole of sodium carbonate provided.
Observation
Inference
(1 mark)
(1 mark)
- To a little amount of Z, add sodium carbonate.
Observation
Inference
(1 mark)
(1 mark)
- Scoop a little of solid Z (using a clean spatula and burn it in a |Bunsen burner flame.
CONFIDENTIAL
Instructions to schools
In addition to the lab fittings and Apparatus found in the laboratory, each candidate should require;
- One burette 0-5ml
- One 20ml pipette
- Three conical flasks
- One volumetric flask
- One complete retort hand.
- One white tile
- One pipette filler
- One test-tube rack
- Six test tubes
- Two boiling tubes
- Filter paper -2
- Filter funnel -1
- Measuring cylinder 100ml-1
- Measuring cylinder 10ml
- Wash bottle – with water (distiled)
- One metallic spatula
- Solution G – 1M HCl
- Solution H – 0.1M NaOH
- Solid/metal F – 0.3g Magnesium powder
- Solid Z – 1 spatula maleic acid in a stoppered container.
- Solid K – Potassium chlorate 2g KClO3
- Solid R – mixture of Al2(SO4)3 and CuSO4 at Ratio of 1:1.(Provide 10cm3 of solution R)
- Thermometer – 10 to 110oc
- 1g Na2CO3 – In a stoppered container
Access to:
- Source of Heat
- Water bath of hot water
- 2MNaOH(aq)
- 2MNH3(aq)
- 2M HNO3
- 2M HCl
- 5m Barium chloride(acidified)
- Acidified Potassium Manganate (VII) solution
MARKING SCHEME
- Table 1……………………………………………………5mks
(i). Complete table ………………………………………..1mk
Conditions
- Complete table with 3 titrations done …..….1mk
- Incomplete table with only 2 titrations ½ mk
- Incomplete table with only 1 titration one ……...0mk
- For no titration done ….…..0mk
NOTE: where NO Titration done penalize FULLY for ALL the marking points for table
Penalties
Before awarding a mark for complete table
The examiner MUST ensure that none of the following mistakes is there in the table – otherwise penalize ½ k for EACH mistake to a maximum penalty of ½ mk i.e penalize ½ mk ONCE even if there are 2 or more mistakes
- Wrong arithmetic/subtraction
- Inverted table
- Burette readings beyond 50.0cm3, except where explained.
- Unrealistic titre values i.e. titres <1.0cm3 or>100cm3.
(ii). Use of decimals (tied to 2nd and 3rd rows ONLY) ……………….1mk
Conditions
Either 1 or 2 decimal places used consistently
If 2 decimal places are used then 2nd decimal place MUST be either 0 or 5
(iii). Accuracy (tied to correct titre values only……………………..1mk
Compare the candidate’s correct titre values with the school values i.e. Teacher’s Average tire
Conditions
- If at least one titre value is ± 0.1cm3 of school value award ……………1mk
- If NO titre value is within ±0.1cm3 0.1cm3 of school value but at least one titre value is within ±0.2cm3 of school value then award ………….1/2 mk
- If NONE of the titre values is within ±0.2cm3 of the SV award …….0mk
(iv). Principles of averaging …….1mk
- If 3 consistent values average -------1mk
- If 3 titrations done,2 consistent values average d……..1mk
- If 2 titrations done , inconsistent and averaged ……0mk
- If 3 inconsistent titrations average …..0
(v) Final accuracy (tied to correct average titred………..1mk)
Compare the candidate correct average tire with the SV and award accordingly
- If within 1cm3 of S.V award …..1mk
- If NOT within ±0.1cm3 of S.V but is within ±0.2cm3 of S,V award ….. ½ mk
- If > ±0.2cm3 of S, V award …… ½ mk
- Complete table ….1mk
- Use of decimals ……1mk
- Accuracies ……1mk
- Principles of Averaging …....1mk
- Final Accuracy ….1mk
Total 5mks
(vi). Calculations
(i). Mols of Hcl is 50cm3 of solution G
= 50 x 1 1/2 = 0.05 moles 1/2
1000
NB units may be given or NOT given but if given MUST be correct
(ii). Moles of NaOH in 25cm3 of solution H
= 50 x 0.1 1/2 = 0.025 moles 1/2
1000
(iii). Mole of HCl in average volume of
Solution Q used.
= mole ratio
NaOH : Hcl =1:1 1/2
= correct newer 1/2
(iv). Moles of Hcl unreacted
= answer (iii) above x 250 = correct answer 1/2
Average tire
OR
= Answer in (i) – Answer in (iii) 1/2
= Correct answer 1/2
(v). Moles of HCl reacted with F
= Answer in (i) = Answer in (iv)
= Correct answer 1/2
(vi). Moles of metal F reacted
= ½ x Answer (v) above 1/2
= Correct answer 1/2
(vii) R.A.M for F
= 0.3 1/2= correct answer
Answer (vi)
Table 2………………………………………….6mks
Distribution.
(a). Complete table ………………3 mks
Conditions/penalties
- Award ½ mk for EACH experiment completely done
- Penalize ½ mk for EACH solubility value either wrongly worked out or NOT worked to maximum of 1mk.
- Penalize ½ mk if ALL temperature readings in the table are CONSTANT.
(b). Use of decimals tied to temperature readings)……………….1mk
- Accept ONLY if all readings recorded consistently either as whole Numbers or to one decimal places of -0 or -5 otherwise penalize FULLY.
(c). Accuracy …………………………1mk
- Compare candidate’s first temperature reading i.e. when 5cm3 of water is added with school value if within 0oc of S.V award 1mk otherwise award 0mk
(d). Trend ……………………..1mk
Award mark for Temperature readings showing a continuous Drop otherwise penalize Fully
- Complete table 3mks
- Use of decimals 1mk
- Accuracy 1mk
- Trend 1mk
06mks
Graph……………………………..3mks
Award 3 mks – distribution
A. Labelling of Axes …………….1/2 mk
Award ½ mk if both axes correctly Tabelled
Penalties
- Penalize FULLY for inversion on axes
- Penalize FULLY for wrong units given, ignore if units are NOT given
- Penalize FULLY if only one axis is labelled
B. Scale …………….1/2 mk
Conditions
- Area covered by the graph plots must be at least 8 big squares on both axes
- Scale interval must be consistent on each axis
- Scale chosen must accommodate all the plots
C. Plotting
Conditions
- If 6 or 5 points correctly plotted award …………..…1mk
- If only 4 or 3 points are correctly plotted award ……1/2 mk
- If less than 3 parts correctly plotted award …….0
D. Curve ………………………….1mk
Award 1mk for a smooth curve which is rising an joining at least three correctly plotted points
- Graph- ….3mks
- Labelling of Axes …..½ mk
- Scale …..1/2 mk
- Plotting …..1mk
- Curve ……1mk
03mks
H. Solubility of K at 25oC using the graph
= correct showing on graph ½
= correct answer ½ mk
Question 2
(a)
|
observation |
Inference |
|
Colourless filtrate Blue precipitate ½ |
Zn2+,Al3+,Pb2+present Cu2+P Present |
(b)
|
observation |
Inference |
|
No effervescence ½ |
½ |
(b.i).
|
observation |
Inference |
|
No white precipitate ½ Dissolves in excess ½ |
Zn2+,Al3+,Pb2+present |
(b)(ii).
|
observation |
Inference |
|
white precipitate ½ dissolves in excess ½ |
Al3+,Pb2+ present |
c)
|
observation |
Inference |
|
No white precipitate ½ No effervescence |
Al3+, present Pb2+ absent 1/2 |
(d).
|
observation |
Inference |
|
white precipitate ½ |
SO42- present 1 ½ |
e).
|
Observation |
Inference |
|
blue precipitat dissolve to form deep blue solution |
Cu2+ in present |
3.(a).
|
Observation |
Inference |
|
burns with yellow sooty/smoky flame 1mk |
|
(b).
|
Observation |
Inference |
|
Dissolve to form colourless solution mk |
Polar substance |
c).
|
Observation |
Inference |
|
Purple potassium manganite (VII) decolorized mk |
|
(d).
|
Observation |
Inference |
|
Effervescence occurs mk |
R – COOH present 1 mk |
Download Chemistry Paper 3 Questions, Answers and Confidential - Arise and Shine Mock Exams 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students