INSTRUCTIONS TO CANDIDATES:
- Write your name and ADM NO in the spaces provided above
- Answer All the questions in the spaces provided below each question
- All working MUST be clearly shown where necessary
- Sign and write the date of examination in the spaces provided above
- Electronic calculators may be used
- Candidates should check to ascertain that all pages are printed as indicated and that no questions are missing
For Examiner’s Use Only
|
Question |
Maximum score |
Candidate’s score |
|
1- 29 |
80 |
QUESTIONS
- State two laboratory rules that should be followed to avoid contamination and wastage of chemicals (2 marks)
- Describe one method used to distinguish between sodium sulphate and sodium sulphite (2 marks)
- A gaseous compound consists of 86% carbon and 14% hydrogen by mass At stp 32 dm3 of the compound has a mass of 6 grams Calculate:
- its empirical formulae ( C=12,H=1, MGV=at stp 224dm3 (2 marks)
- its molecular formulae (2 marks)
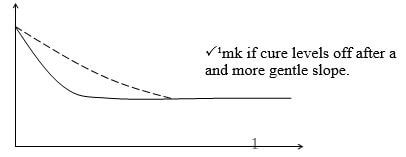
- The graph below shows amount of calcium carbonate and calcium chloride varying with time in the reaction
CaCO3 + 2 HCl → CaCl2 + H20 + CO2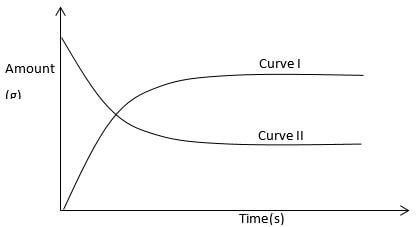
- Which curve shows amount of calcium chloride varying with time (1 mark)
- Explain why the two curves become horizontal after given period of time (1 mark)
- Sketch on graph curve II would appear if the experiment was repeated using more dilute hydrochloric acid solution (1 mark)
- The thermal chemical equation for the reaction between X and Y are shown below
2X2 (g) + Y2(g) ―—→ 2X2Y(g) ∆H= -197KJ/mol- Other than change in temperature, suggest two ways in which yield of X2Y can be increased (1 mark)
- draw a well labeled energy level diagram for the forward reaction (2 marks)
- The diagram below was used by a form two student at Kimuchul secondary school to prepare and collect dry carbon IV oxide
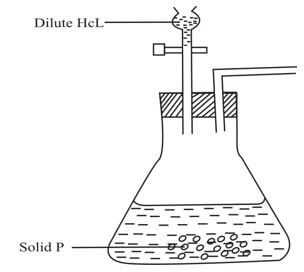
- Name suitable solid p (1 mark)
- Complete the diagram above (3 marks)
- The melting point of Nitrogen is -1960c while that of sodium is 980 c In terms of structure and bonding explain the difference in the melting point of Nitrogen and sodium (2 marks)
- The following tests were carried out on three separate portion of colourless solution P
TEST
OBSERVATION
i)Addition of dilute hydrochloric acid to the first portion of solution P
Formation of effervescence
ii)Addition of aqueous potassium sulphate solution to the second portion of solution P
No white precipitate
iii)Addition of acqueous sodium hydroxide to the third portion to portion of solution P till in excess
White precipitate formed which dissolved in excess to form colorless solution
- From information in test(i),name two anions that are likely to be present in solution P (1 mark)
- Identify cations that are likely to be present in solution P (1 mark)
- Write an ionic equation for the reaction which takes place in test (i) (1 mark)
- Describe how you would prepare crystals of potassium sulphate starting with 100cm3 of 05M Potassium hydroxide (3 marks)
- use dot() and cross(x) diagram to show bonding in magnesium fluoride (Mg=12 , F=9) (1 mark)
- An element R has a relative atomic mass 88 When a current of 05A were passed through fused chloride of R for 32 minutes and 10 seconds, 044g of R were deposited at cathode Determine charge of ion Q (I F– 96500C) (3 marks)
-
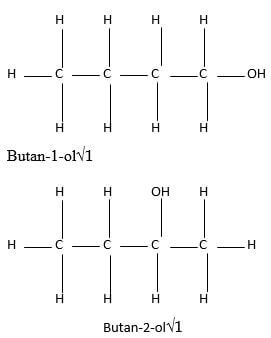
- Define the term isomerism (1 mark)
- Draw and name two positional isomers of butanol (2 marks)
- During the extraction copper and zinc from their ores, some processes include:
- Crushing (1 mark)
- Mixing of the crushed one with oil and water and bubbling air through it
- Name the process (b) above (1 mark)
- What is the purpose of(b) above (1 mark)
- Write an equation when the following compounds are heated
- zinc nitrate (1 mark)
- silver nitrate (1 mark)
- The table below gives solubilities of potassium bromide and potassium sulphate at 00c and 400c
When an aqueous mixture containing 60g of potassium bromide and 7g of potassium sulphate in 100g of water at 40ºc was cooled to 0ºc some crystals were formedSolubility g/100g H2O at
00c
400c
Potassium bromide
55
75
Potassium sulphate
10
12
- identify the crystal (1 mark)
- Determine the mass of the crystal (1 mark)
- The set up below was used to carry out electrolysis of a molten bromide of metal X,XBr2
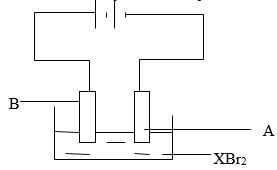
- write equations for reaction taking place at:
- Anode : (1 mark)
- Cathode (1 mark)
- Give reason why experiment should be carried out in fume chamber (1 mark)
- write equations for reaction taking place at:
- below are the standard electrode for electrodes X and Y
X2+ (aq )+ 2e __________________ X(s) -292 V
Y2+ (aq ) + 2e _____________________ Y(s) + 034 V- identify the electrode which is the least reducing agent (1 mark)
- calculate the emf of the cell formed when the two electrodes are connected (2 marks)
- write cell representation for the cell above (1 mark)
-
- Give two differences between a nuclear reaction and a chemical reaction (2 marks)
- complete the nuclear equation below (1 mark)
230 230
90Th ——→ 92 Pa +
- Explain why the boiling point of ethanol is higher than that of hexane (Rmm of ethanol 46 while that of hexane is 86 (2 marks)
- The atomic number of element Q is 8 and that of P is 11
- Write down the formulae of compound formed between Q and P (1 mark)
- Name the type of bond formed by compound given in (a) above Explain (2 marks)
-
- State charles’ law (1 mark)
- sketch a graph to represent Charles law (1 mark)
- A gas occupied a volume of 250cm3 at -23ºc and atmosphere Determine volume at 107 ºc when pressure kept constant (2 marks)
- The structures shown below represent two cleansing agent A and B
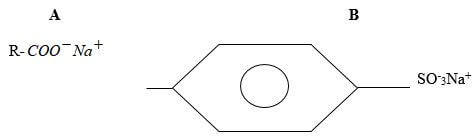
- Name the type of cleansing agent A (1 mark)
- Which of the two cleansing agents is more suitable for washing in water containing calcium chloride? Give a reason (2 marks)
-
- Define the term enthalpy of formation of a compound (1 mark)
- Use the information below to answer the questions that follow:
Equation Enthalpy of formation- H2(g) + ½ O2(g) → H2O(l) ∆H1= -286kJmol-1
- C(s) + O2(g) → CO2(g) ∆H2= -394kJmol-1
- 2C(s) + 3H2(g) + ½ O2(g) → C2H5OH(l) ∆H3= -277kJmol-
Calculate the molar enthalpy of combustion of ethanol Given that:
C2H5OH (l) + 3O2 (g) → 2CO2 (g) + 3H2O (l) (3mks)
- The chromatogram below shows the constituents of a flower extract Study it and answer the questions that follow
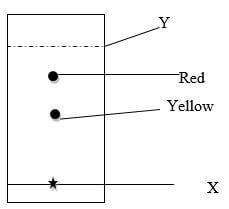
- Explain the different positions of red and yellow pigments (2 marks)
- Describe how the solid yellow pigment can be obtained in the chromatogram (2 marks)
- When excess chlorine gas is bubbled through cold dilute sodium hydroxide solution, the resulting solution acts as a bleaching agent
- Write an equation for the reaction between chlorine gas and sodium hydroxide solution (1 mark)
- Explain how the resulting solution acts as a bleaching agent (2 marks)
- The table below gives some properties of four substances Study it and answer the questions that follow:
Substance
M.P 0C
B.P 0 C
Electrical Conductivity
Solid
Liquid
W
1723
2230
Poor
Good
X
993
1695
Poor
Poor
Y
- 183
- 164
Poor
Poor
Z
1083
2567
Good
Good
- Which substance is suitable for making cooking pans? Explain (11/2 marks)
- Which substance is likely to have a giant atomic structure? Explain (11/2 marks)
- 17g of Zinc carbonate was reacted with 50 cm3 of 4M nitric acid Calculate the mass of Zinc Carbonate that remained unreacted (Zn = 65, C = 12, O = 16) (3 marks)
- A certain element Z forms an ion of type Z3- If the element is in period 3
- Write the electronic configuration of Z3- (1 mark)
- How do the sizes of Z and Z3- compare Explain your answer (2 marks)
- A sample of copper turnings was found to have contaminated with copper II oxide Describe how a sample of copper metal can be separated from the mixture (2 marks)
MARKING SCHEME
- Label all the chemicals to avoid confusion.
Always use a clean spatula/dropper for scooping/droping substance from container
Chemicals already used must be disposed off safely to avoid condamination.
Read container labels carefully to avoid mixing the wrong chemicals . - Add acidified barium chloride /nitrate ,a white precipitate will be formed with sodium sulphate and no white precipitate with sodium sulphite
-
-
E.F = CH2element
C
H
Mass in %
86
14
RAM
12
1
No. of moles
86/12=7.1 ½
14/1=14 ½
Mole ratio
1
2
- its molecular formulae (2mks)
6g of CH2 occupies 3.2dm3
22.4dm3
22.4x 6 = 42
3.2
(E.F)n =M.F
(CH2)n=42
14n=42
n=3
MF = C3H6
-
-
- CURVE II
- The reaction will have reached completion and the amount of reactants and products do no change further.
-
- Increasing the pressure ½
Decreasing the temperature ½
Adding more of X2 and Y2
Removing X2Y formed 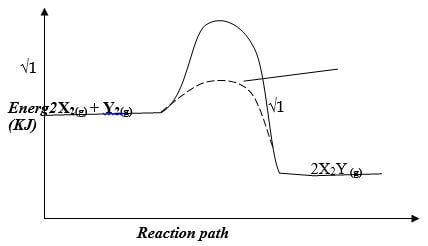
- Increasing the pressure ½
-
- Calcium carbonate or any carbonate except lead carbonate
- Complete the diagram above (3mks)
Passing through a suitable labeled dryng agent i.e conc sulphuric acid ;calcium oxide ;anhydrous chloride 1 mk
Correct method of collection i.e downward delivery 1mk
Workability 1mk
- Molecules of nitrogen are held by a weak van der waal forces1 while that of sodium has metallic bond thoughout the structure
-
- Sulphate ions ½
Carbonate ions ½ - Alluminium ions ½
Zinc II ions ½ - CO32- (aq) +H+ (aq) → CO2(g) +H2O(l) ║ SO32- (aq) + H+(aq) →SO2 (g) +H2O(l)
- Sulphate ions ½
-
- Add 100 cm3 of 1 M sulphuric acid or 50 cm3 of 0.5 M of sulphuric acid to 100cm3 of 0.5 M KOH
- evaporate the resulting solution to saturate and allow it to cool
- filter the mixture and dry the residue between the filter papers 1
NOTE. The volume and the concentration of sulphuric acid must be mention.
Otherwise penalize fully
- Q=IT
O.5X [(32X60) +10]= 965C
0.44g of R = 965C
88g of R =
88x965 =193000c
0.44
1F=96500C
= 193000C
(19300X1 ) = 2F1
96500
charge = 2+ penalize ½ mk if +ve sign is not shown after the number. -
- ability of the compound to have the same molecular formulae but different structural formulae
-
- Increasing the surface area
-
- Froth flotation
- To concentrate the ore
-
- 2Zn(NO3) 2 (s) → 2ZNO (s) + 4NO2(g) +O2 (g)
- 2AgNO3(s) →2Ag(s) + 2NO2(g) + O2(g)
-
- potassium bromide
- 60-55= 5g
-
-
- A : X2+ (aq) + 2e → X (s)
- B… Br- (aq) → Br2 (g) + 2 e
- There is production of poisonous bromine gas notes. The gas must be mention
-
-
- Y1
- E cell= E red – E oxid
+0.34- - 2.92 1 penalise ½ mk if the sign and unit is missing
= +3.261 - X (S)│X2+(aq)║Y2+(aq) |Y (s) 1
-
- nuclear reacstion produces large amount of heat while in chemical reaction produces less amount of heat
In nuclear reaction it involves only the protons and neutrons while a chemical reaction involves the valence
In nuclear reaction are not affected by environmental factors while in chemical reactions are affected by the environmental factors - 230 230
90Th ——→ 92 Pa + 2 -10e
- nuclear reacstion produces large amount of heat while in chemical reaction produces less amount of heat
- Hexane molecules are held by weak van der waal forces 1while ethanol molecules are held by hydrogen bond
-
- P2Q
- Ionic bond 1.it involves complete transfer of electrons from P to Q
-
- the volume of a fixed mass of a gas is in directly proportional to the absolute temperature at constant pressure 1
- V1=250
T1= -23+273=250K
V2=?
T2=107+273=380K
V2 = V1XT2 = 250X3801 =380CM31
T1 250
-
- Soapy detergent
- B1 – it doesn’t form scum
-
- Enthalpy change that occur when a compound is formed from its constituent element.
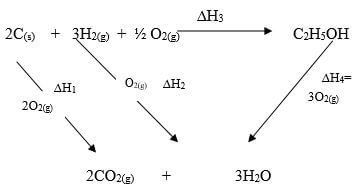
∆H1 + ∆H2 = ∆H3 + ∆H4
Then
∆H3 = ∆H1 + ∆H2 - ∆H4
∆H3 (2x-394) + (3x -286)-(-277) 1
=-788 + 853- -277
= -788 – 853 + 277
∆H3 =-1646+277 =-1369∆H3 = -1369kJ/Mol1
-
- R is more soluble than Y
-
- cut the yellow spot and placed in a beaker containing ethanol/methanol/propanone and allow it to dissolve.
- remove the cut part of paper from the beaker when all yellow pigment has dissolve and leave in the sun to evaporate ethanol/methanol/propanone used .
-
- 2NaOH(aq) + Cl2(g) → NaOCl(aq) + NaCl(aq) + H2O(L)
- The resulting mixture contains NaOCl which decomposes to give oxygen which bleaches by adding itself to the dye
-
- Z ½- it has high M.P and B.P ½. and also conductor of electricity in solid and liquid state ½
- X ½—has a high M.P and B.P ½ and do not conduct electricity in solid and liquid state ½
- ZnCO3 + 2HNO3 →Zn(NO3)2 +H2O +CO2
50x4= 0.2 moles .
1000
Mole ratio of HNO3 : ZnCO3 = 2:1
Moles of ZnCO3 = 0.2/2 = 0.1moles
Mass of ZnCO3 = no. of moles x RMM
0.1x125 =12.5g
Mass of unreacted ZnCO3 = 17-12.5
4.5g -
- 2.8.8
- Z3- has a larger size than Z –since there is repulsive force between the existing electron and incoming electrons
-
- Add excess dilute hydrochloric acid /sulphuric acid to the mixture to dissolve copper II oxide .
- Filter the mixture to obtain copper turnings as a residue
- Wash the residue wih distilled water to remove impurities.
Download Chemistry Paper 1 Questions and Answers - Sunrise 2 Evaluation Mock Exams 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students
