QUESTIONS
- The grid below shows a section of the periodic table. The letters do not represent the actual symbols of the elements.
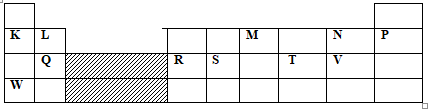
- Name the family to which element P belongs.(1mk)
- Which two elements will form carbonates that do not decompose on heating ( 2mks)
- With a reason, identify an element in period 3 with the largest atomic radius ( 2mks)
- Write the formula of the compound formed between L and M ( 1mk)
- State two uses of element R and for each use , state property of element R that makes it possible for the use
- Use (1mk)
Property(1 mk) - Use (1mk)
Property(1 mk)
- Use (1mk)
- Using dots (.) and cross (x), show bonding in the compound formed between R and oxygen( 2 mks)
- In terms of structure and bonding explain why the oxide of element V has relatively low boiling points( 2mks)
-
- Name the following compounds ( 3mks)
- CH3CH2CH2COOH
- H2C Br– CH(CH3) – CH2 – CBr = CH – CH3
- CH3CH2COOCH2CH3
- Two types of detergents P and Q can be represented as
P;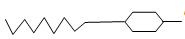 OSO3-Na+
OSO3-Na+
Q; COONa+
COONa+- Identify each type of the detergent ( 2mks)
- Which of the two detergents is the best to use with hard water? Give reason. ( 2mks)
- State one disadvantage of detergent P( 1mk)
- State advantage of detergent Q( 1mk)
- A compound is represented as shown below
CH3CH2CH2COOC2H5- Name the compound. ( 1mk)
- Name two reagents that can be used to generate the compound( 2mks)
- Name the following compounds ( 3mks)
-
- Define the term electrolysis ( 1mk)
- State two function of the salt bridge during electrolysis (2mks)
- During the electrolysis of a molten chloride of metal Q, a current of 0.25A was passed through the molten chloride for 2 hours and 10 minutes .Given that 0.9 g of metal Q were deposited at the cathode.
- Calculate the quantity of electricity passed (1mk)
- Charge carried by the ions of metal Q given that R.A.M of metal Q is 84 (3 mks)
- Electric current was passed through a concentrated solution of copper (II) chloride as shown in the diagram below.
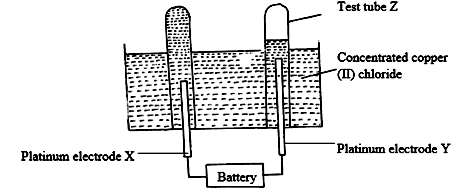
- Write the chemical equation for the reaction at the cathode? (1 mark)
- After sometime test-tube Z was found to contain a mixture of two gases. Explain this observation. (2 marks)
- State the observations that would be made at the anode if the platinum electrodes are replaced with copper electrodes. (2 marks)
- State one application of electrolysis in iron industry (1mk)
-
- Define the term saturated solution. (1mk)
- Solubility of salt X and Y were determined at different temperatures as shown in the following data.
Temperature (ºC) 0 20 40 60 80 100 Solubility of 100g of water X 12 30 75 125 185 250 Y 15 20 35 45 65 80 - On the grid provided, plot a graph of solubility (vertical axis) against temperature. (4mks)
- From the graph determine the solubility of each at 50ºC.
X (1mk)
Y (1mk) - At what temperature was the solubility of both salts equal? (1mk)
- What is permanent hardness of water? 1mk)
- The diagram below shows an experiment incorrectly set-up to investigate a property of carbon (ii) oxide. Study it and answer the questions that follow.
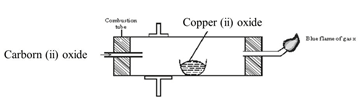
- Name one condition that is missing in the set up that must be present if the experiment to proceed. (1mk)
- If the experiment was carried out properly, what observation would be made in the combustion tube?(1mk)
- Give a name for the type of reaction that occurs in the combustion tube. (1 mk)
- Write an equation for the reaction that takes place as gas x burns. (1 mk)
- Why is it necessary to burn gas x? (1mk)
- Name the reducing and oxidizing agent. (2mks)
- Reducing agent
- Oxidising agent
- Identify any other substance that would have the same effect on copper (II) oxide as carbon (II) oxide. (1mk)
- What would happen if copper (II) oxide was replaced with sodium oxide? Explain. (2mks)
- Dry chlorine was collected using the set up below.
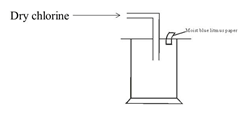
- Name a suitable drying agent for chlorine gas? (1mk)
- State one property of chlorine gas which facilitates this method of collection. (1mk)
- State the observations made on the moist blue litmus paper. Explain. (2mks)
- Chlorine gas was bubbled through distilled water. With aid of an equation show the formation of chlorine water. (1mk)
- Write the formula of the compounds formed when chlorine gas reacts with warm dry phosphorous. (2mks)
- Chlorine gas is mixed with moist hydrogen sulphide gas. State and explain the observations.(2mks)
- Give one use of chlorine gas. (1mrk)
- A metal F is very reactive and therefore it is extracted by electrolysis of its fused chloride. The electrolytic cell used in its extraction is made of anode surrounded by a ring shaped iron cathode enclosed in a wire gauze shell that acts as a partition separating the two electrodes.When exposed to air it loses its lustre.At 620°C, it reacts with liquid ammonia liberating hydrogen gas.It is used as a deoxidizing agent in the preparation of light alloys and some rare earth metals from their oxides.
-
- Name the process by which metal F is extracted. (1mk)
- What is the identity of metal F. (1mk)
- State the name of the ore from which metal F is extracted. (1mk)
- Explain why the metal loses its lustre when exposed to air. (1mk)
- What is the function of wire gauze shell that separates the anode from the cathode? (1mk)
- Write a chemical equation for the reaction between metal F and ammonia(1mk)
- Apart from being a deoxidizing agent, state two other uses of metal F. (2mks)
- During extraction of aluminium by electrolysis, molten cryolite is used instead of water and the anode must be replaced from time to time.
- State the main ore from which aluminium is extracted (1mk)
- Explain why cryolite is preferred over water (1mk)
- Give a reason why the anode is replaced from time to time. (1mk)
- Extraction of aluminium is very expensive compared to other metals like Iron, explain (1mk)
-

MARKING SCHEME
-
- Noble gases reject rare/inert gases
- K and W accept Lithium and Potassium
- Q, hs the fewest number of protons hence experiences weaker nuclear force of attraction.
- L3M2
- Making electric cables: it is a good conductor of electricity, it is ductile.
- Making cooking pans: It is malleable good conductor of heat.
- R2O3
- Hassimple molecular structure with weak van der waals forces holding the molecules together.
-
-
- Butanoic acid
- 3,6-dibromo-5-methylhex-2-ene
- ethylpropanoate
-
- P - Soapless detergent
Q- Soapy detergent. - P, does not form scum/ lathers easily with hard water.
- Non- biodegradeable hence pollutes the environment// exppensive
- Biodegradeable hence does not pollute the environment// cheap
- P - Soapless detergent
-
- ethylbutanoate
- butanoic acid& ethanol
-
-
- Decomposition of an electrolyte by passing an electric current through it.
- completing the circuit
maintain electrical neutrality within internal circuit. -
- Q=it
= 0.25 x 7210
=1802.5 - 0.9→1802.5
84→?
48 x 1802.5
0.9
= 168,233.3333
1 charge→96500 C
? →168,233.333
168,233.333
96,500
= 1.7434
= +2
- Q=it
-
- Cu2+(aq) + 2e → Cu(s)
- At the beginning, OH-ion are preferentially discharged at the anode where they lose electrons producing O2 gas. After sometime the concentration of OH- decreases and Cl- are preferential discharged due to their higher concentration, they lose electron producing Cl2 gas.
- Anode dissolves in the solution, copper anode is an active electrode.
- Electroplating to prevent rusting and improve its appearance
-
- A solution in which no more solute can dissolve at a particular temperature.
-
-
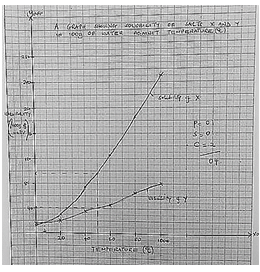
- X = 96g/100g of water ±2g
Y = 41g /100g of water ±2g - 7˚C ±1°C
-
- Hardness that cannot be removed by boiling/ hardness caused by dissolved Mg2+ or Ca2+ ions carnonates.
-
- Heat/heating Cuo.
- Black Cuo turns /changes to reb-brown copper metal.
- Redox
- 2CO(g) + O2(g)→ 2CO2(g)
- It is poisonous.
-
- carbon (II) oxide
- Copper (II) oxide
- Ammonia gas // Hydrogen gas (any one)
- White ash of Na2O remains white// no change ; Na is above C in the reactivity series hence CO cannot reduce Na2O.
-
- Concentrated Sulphuric (VI) acid.
- Denser than air.
- turns red then eventually bleached to white. Turns red because solution is acidic, turns white because it is bleached by HOCI
- Cl2(g) + H2O(I)→HOCl(aq) + HCl(aq)
- PCl5
PCl3 - Yellow deposits of sulphur; chlorine oxidizes to H2S to S and itself is reduced to hydrogen chloride’
- Treatment of water (accept any other)
-
-
- Downs process
- Sodium
- Rock salt
- Reacts with oxygen , nitrogen and hydrogen carbonates in air forming a coat on the surface.
- Prevents Cl2(g) from combining with Na(l)
- 2Na(s) + 2NH3(L) →620°C→ 2NaNH2(L) + H2(g)
- Combined with cyanide used in extraction of gold. etc
-
- Bauxite
- Bauxite is insoluble in water but soluble in cryolite
- O2 produced reacts with carbon anode.
- A lot of electricity is used in its extraction.
-
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 2 Questions and Answers - Kakamega Evaluation Mock Exams 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students
