QUESTIONS
-
- Study the table below and answer the questions that follows. The symbols are not the actual symbols of the elements
Element A B C D E F Electronic configuration of the ion 2.8 2.8.8 2.8 2.8.8 2.8 2.8.8 Valency and type of ion Divalent cation Divalent anion Monovalent cation Monovalent anion Trivalent cation Trivalnet anion - Arrange the elements A,B,C,D,E and F in terms of increasing atomic sizes (1mk)
- Name the period of the periodic table to which these elements belongs? (1mk)
- Write an equation for the reaction between elements C and D (1mk)
- Compare the electrical conductivity of elements A and E. Explain (2mks)
- Compare the first ionization energies of elements A and C. Explain. (2mks)
- Which element sit re strongest oxidizing agent (1mk)
- Study the information below and answer the question that follow
Aluminum chloride(AlCl3) has an unexpected bond type and structureFormula of oxide NaCl MgCl2 AlCl3 SiCl4 PCl3 SCl2 Melting point ºC 801 714 - -70 -97 -80 Formula of oxide Na2O MgO AlO3 SiO2 P4O10 SO2 Melting point ºC 1190 3080 2050 1730 560 -73 - State the type of bond and the structure in AlCl3 (1mk)
- What type of bond would AlCl3 be expected to have? Give a reason (1mk)
- Why is the melting point of AlCl3 not indicted in the table above (1mk)
- Silicon (iv) chloride get hydrolyzed by water. Write a balanced equation for this reaction(1mk)
- Study the table below and answer the questions that follows. The symbols are not the actual symbols of the elements
- The following flow chart is an illustration of the extraction of aluminum from its ores
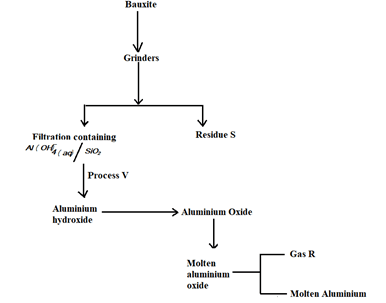
- Give the formula of bauxite (1mk)
- Name the two main impurities in bauxite (2mks)
- Write the chemical equation for the reaction taking place during process V (1mk)
- The melting point of aluminum oxide is quite high. Explain what is done to make the process cost effective. (1mk)
- Name residue S Write down the half equations for the reaction at the (2mks)
- In the electrolysis stage the graphite electrode used at the anode is periodically replaced anode at regular intervals (1mk)
- Give two properties that makes aluminum and alloys suitable for making aircraft bodies (2mks)
-
- A form four students carried out an experiment to extract oil from sim sim seeds. (1mk)
- Name two apparatus he used to crush the simsim seeds (1mk)
- Name suitable solvent used (1mk)
- Name the class or organ compounds in which vegetable oil belongs to (1mk)
- Describe the process of preparing soapy detergents using vegetable oil (3mks)
- Study the flow chart below and answer the question that follow
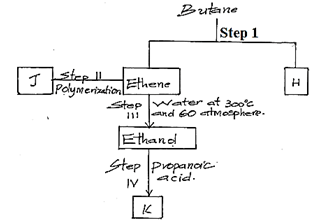
- State the conditions for the reaction in step 1 to occur (1mk)
- Identify substance H (1mk)
- Give one disadvantage of the continued use of substances such as J (1mk)
- The relative molecular mass of J is 16800. Calculate the number of monomers that make up J (2mks)
- A form four students carried out an experiment to extract oil from sim sim seeds. (1mk)
- Study the standard electrode potentials of the half – cells given below and answer the questions that follow. The letters do not represent the actual symbols of the elements)
EƟ Volts
N+(aq) + e- ⇌ N(s) -2.92
J+(aq) + e- ⇌ J(s) +0.52
K+(aq) + e- ⇌ ½K2(g) 0.00
G+(aq) + e- ⇌ G(s) +0.80
M2+(aq) + 2e- ⇌ M(s) -0.44- Identify the strongest oxidizing agent. Give a reason for your answer (2mks)
- Which two half- cells would produce the highest potential difference when combined (1mk)
- Draw an electrochemical cell for the above. Show on the diagram flow of electrons (3mks)
- Write the cell notation representation for the above cell (1mk)
- Calculate the e.m.f of the cell above (1mk)
- Give two functions of the salt bridge (2mks)
- Determine the oxidation number of the halogen in I04 - (1mk)
- Give on use f electrolysis (1mk)
-
- The table below shows the observations made when aqueous ammonia and sodium hydroxide solutions were added to cations of A,B,C and D
Cation of Sodium hydroxide in excess Ammonia solution in excess A Blue precipitate Blue precipitate dissolves to form a deep blue solution B White precipitate dissolve to form a colourless solution White precipitate dissolve to form a colourless solutuion C White precipitate dissolved to form a colourless solution White precipitate D - Identify the cations present in (2mks)
- Write the formula of the complex ion present in the deep blue solution of cation A (1mk)
- Given that the cation of D have Na + ions complete the table above (1mk)
- State any one use of complex ions (1mk)
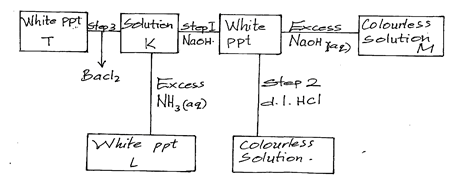
- Name precipitate L (1mk)
- Write the ionic equation for the formation of L (1mk)
- Name the type of reaction in step 2 (1mk)
- Name any other solution that can be used in step 2 above (1mk)
- The table below shows the observations made when aqueous ammonia and sodium hydroxide solutions were added to cations of A,B,C and D
- The diagram below was used to prepare hydrogen chloride gas which was passed over heated iron powder
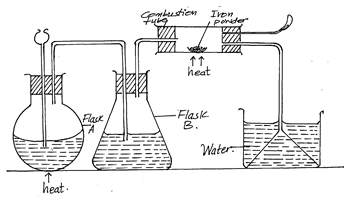
-
- State a pair of reagents that will produce hydrogen chloride gas in flask A (1mk)
- Name the substances in flask B (1mk)
- State the observations made in the combustion tube (2mk)
- Write the equation for the reaction in the combustion tube (1mk)
- Describe a chemical test for hydrogen chloride gas (2mks)
-
- Identify the gas that burns at the jet (1mk)
- Explain why the gas in (b) is burnt (1mk)
- Another experiment was carried out where hydrogen chloride gas was bubbled through methylbene and water in separate beakers the resulting solutions were tested with blue litmus paper and marble chips(2mks)
Solution of hydrogen chloride gas Blue litmus paper Marble chips Water Methylbenzene
-
- The thermodynamic equation for the formation of ammonia in the Harber process is as follows.
- If the system is allowed to attain equilibrium. State and explain how the following factors would affect the yield of ammonia
- Increase in temperature (1mk)
- Increase in pressure (1mk)
- Using a more efficient catalyst (1mk)
- In an experiment to study the rate of reaction 1g of Magnesium ribbon was reacted with excess 2M hydrochloric acid. The results obtained were recorded as shown in the table below.
Time(s) 0 20 40 60 80 100 120 140 160 180 Volume of gas produced (cm3) 0 11 20 26 31 35 38 39 40 40 - Give a reason why Magnesium ribbon is normally cleaned with sand paper before being put into the acid (1mk)
- Write a balanced chemical equation for the reaction (1mk)
-
- On the grid provided plot a graph of volume of the gas produced against the time taken (3mk)
- From the graph determine the rate of reaction at
- 30 seconds
- At 120 seconds
- Give a reason for the difference between the two values
- If the system is allowed to attain equilibrium. State and explain how the following factors would affect the yield of ammonia

MARKING SCHEME
-
-
- D, B,F,E,A,C
- Period 3
- 2C(s) + D2(g) → 2CD (aq)
- E is better conductor than A. E has 3 delocalized electrons per atom compared to A which has one delocalized electron per atom
- A has lower first ionization energy than C A has a bigger/ lager atomic radius than C
- Element D
-
- Covalent, simple molecule structure
- Ionic bond, compound of a metal and a non-metal
- Sublimes when heated
- SiCl4(l) + 4H2O(l) → SiO2(s) + 4HCl(aq)
-
-
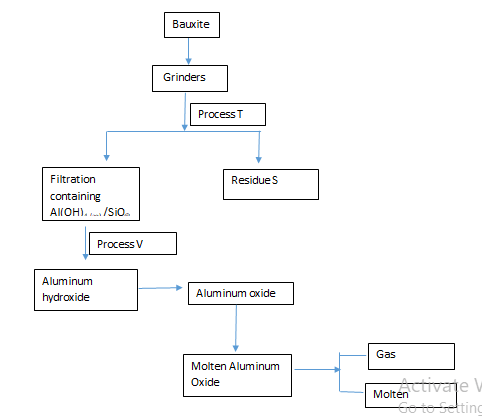
- Al2O3 . 2H2O
- Iron (III) oxide
Silicon (iv) oxide - Al(OH)-4 aq) → Al(OH)3 Al(OH)3(s) + OH-(aq)
OR
[Al(OH)4]- (aq) + CO2(g) → 2Al(OH)3 + CO32-(aq) + H2O(l) - Adding cryolite (Na3 ALF6 ) which lowers the melting point of aluminium, oxide from 2015 to 800ºC
- Residue S – Iron (III) Oxide
Gas R – Oxygen -
- Anode : 6O2- (l) → 3O2(g) + 12e-
- Cathode : 4Al3+(l) + 12e- → 4Al (s)
- The oxygen evolved at the anode reacts carbon electrode to form carbon (iv) oxide. This corrodeS the carbon anode hence the need to replace from time to time
-
- Lighter
- Stronger
- More resistant to corrosion
- Higher tensile strength
-
-
- Mortar and pestle
- Propanone
- Esters
-
- Place vegetable oil in a beaker
- Add NaoH to the oil and stir
- Boil the mixture while stirring
- Add Nacl/sodium chloride solution to the mixture to precipitate soap
- Filter the mixture to obtain soap as residue
-
- 400ºC – 700ºC, Nickel catalyst
- Identify substance H (1mk)
- Ethane
- Non – biodegradable hence pollutes the environment
- 16800/28 = 600
-
-
- G+ has the highest positive electrode potential/Greatest tendency to gain electrons
- N+(aq) + e- → N(s), G+(aq) + e- → G(s)
-
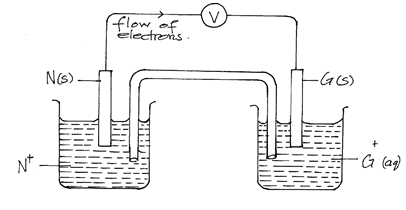
- N (s)/ N+(aq)// G + (aq)/ G(s)
- EƟ = 0.8- -2.92 = + 3.72V
- Complete the circuit by making contact between the electrolytes
Maintain balance o charges / ions on both half cells - x + -8 = -1
x = +7 -
- Electroplating
- Extraction of reactive metals
- Manufacture of pure substance
- Purification of metals
-
-
- B- Zn 2+
C- Pb2+ and Al3+ - [Cu(NH3)4]2+
- No white precipitate
No white precipitate - Softening hard water
Extraction of reactive metals e.g. Al from its ore
- B- Zn 2+
-
- Aluminum hydroxide
- Al3+(aq) + 3OH-(aq) → Al(OH)3(s)
- Neutralisation
- Al(OH)3(s) + 3HCl(aq) → AlCl3(aq) + 3H2O(l)
- Dil sulphuric (VI) acid/ Nitric (v) acid
-
-
-
- Sodium chloride and concentrated sulphuric (vi) acid
- Concentrated sulphuric (VI) acid
- Grey iron powder turns green
- Fe(s) + 2HCl(g) → FeCl2(s) + H2(g)
- Dip a glass rod in concentrated ammonia solution and expose it to HCl(g)
- White dense fumes are produced
-
- Hydrogen/ H2
- Avoid explosion because its mixture with oxygen burns explosively when ignited
Solution of hydrogen chloride gas Blue litmus paper Marble chips Water Turns red Effervescence Methylbenzene Remains blue No effervescence
-
- N2(g) + 3H2(g) ⇌ 2NH3(aq) ΔH = -92KJ/mol
-
- The reaction is exothermic : increase in temperature will favor an endothermic reaction/ backward reaction
- Increase : increase in pressure favors the side with fewer number of molecules / moles / volumes hence more NH3 will be produced
- No effect
-
- Remove the oxide layer
- Mg(s) + 2HCl(aq) → Mg Cl2(aq) + H2(g)
-
- Graph
30 - 7.5 = 0.45cm 3/s
60 - 10 - 45 - 36 = 0.1cm 3/ s
190-100 - Rate of reaction at 30 s higher than at 120s
The concentration of reactants decreases as the reaction continues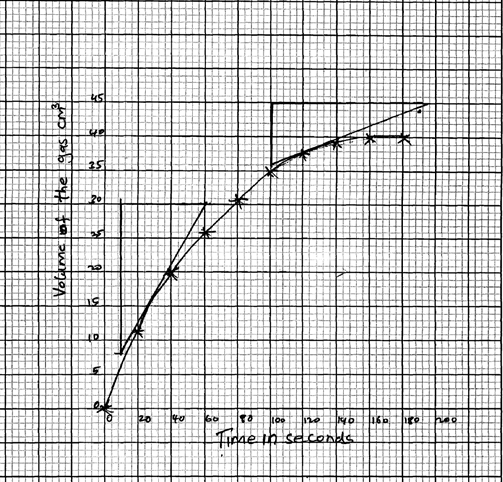
- Graph
-
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 2 Questions and Answers - Cekenas Mock Exams 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students
