Questions
Instructions To Candidates
- Answer all the questions in the spaces provided in the questions paper.
- Mathematical tables and silent electronic calculators may be used.
- All working must be shown where necessary.
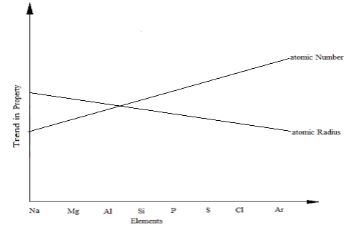
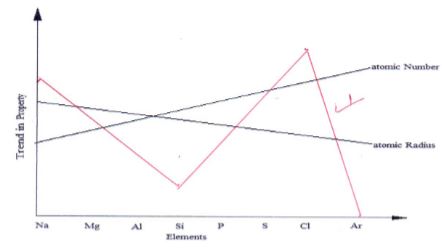
- The figure below represents trends of some properties of period three elements. Study it answer the questions that follow.
- Explain the trends shown by the atomic numbers and the atomic radii
- Atomic number (1 mark)
- Atomic radii (2 marks)
- On the same axes, sketch the trend of reactivity across the period (1mark)
- Write down the electronic configuration of phosphorous and sulphur in the following compounds
- H3PO4 (P=15) (1 mark)
- Na2S2O3 (S=16) (1 mark)
-
- One of the elements given in the figure above is stored under water.
Identify the element and give a reason as to why it is stored under water (1 mark) - State one use of aluminium that can be associated with its malleability. (1mark)
- One of the elements given in the figure above is stored under water.
- Explain the observation that would be made if the chloride of Phosphorous is exposed to moist air. (2 marks)
- Explain the trends shown by the atomic numbers and the atomic radii
-
- Sulphur is extracted from sulphur beds below the earth’s surface. Super-heated water is pumped down a pipe into the sulphur beds.
- What is super-heated water and how is it obtained? (2marks)
- Why does the water used here have to be superheated, and not use boiling water? (1mark)
- When molten sulphur is pumped to the surface, it solidifies. Which allotrope of sulphur forms first? (1mark)
- Name the form of sulphur obtained when liquid sulphur is poured into a beaker of cold water (1mark)
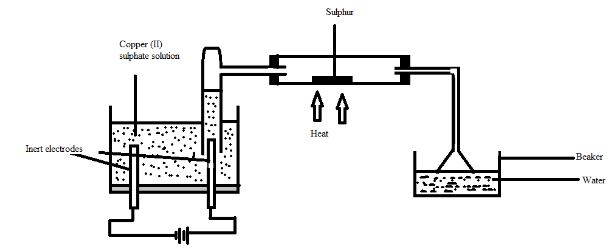
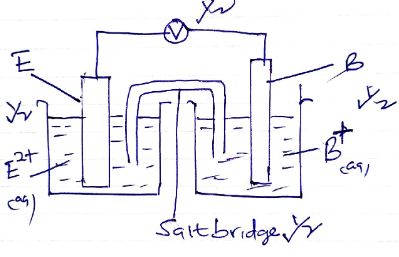
- The diagram below represents a set up that can be used for electrolysis of aqueous copper (II) sulphate. Use it to answer the questions that follow.
- What do you understand by the term inert electrode? (1mark)
- What is the purpose of the filter funnel? (1mark)
- Explain what happens to the pH of the:
- Water in the beaker. (1mark)
- Copper (II) sulphate solution (1mark)
- Write ionic equation for the:
- Oxidation reaction (1mark)
- Reduction reaction, in above set up. (1mark)
- Sulphur is extracted from sulphur beds below the earth’s surface. Super-heated water is pumped down a pipe into the sulphur beds.
-
-
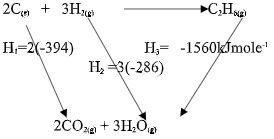
- Define the term ‘molar enthalpy of formation of a compound; (1mark)
- Calculate the molar enthalpy of formation of ethane using the following information:
C2H6(g) + 7 2O2(g) → 2CO2(g) + 3H2O(l), ∆Hfϴ = -1561 kJ/mole
C(s) + O2(g) → CO2(g) , ∆Hfϴ = -394 kJ/mole
H2(g) + 12O2(g) → H2O(l) , ∆Hfϴ= -286 kJ/mole
(3marks)
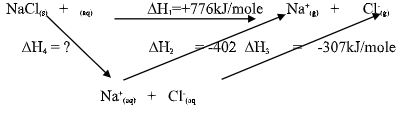
- Study the reactions below and answer the questions that follow:
ΔH
NaCl(s) → Na+(g) + Cl-(g) ΔH = +776 kj/mol
ΔH
Na+(g) → Na+(aq) ΔH = -402 kj/mol
ΔH
Cl-(g) → Cl-(aq) ΔH = -37 kj/mol
ΔH
NaCl(s) → NaCl(aq)- State the name given to :
- ∆H1 ……………………………………………………….(1mark)
- ∆H2 ………………………………………………………(1mark)
- Draw an energy cycle diagram illustrating the reactions above. (2marks)
- Determine the value of H4 (2marks)
- Joy placed 100cm3 of 0.1M CuSO4(aq) in a plastic beaker , covered it with some cotton wool and recorded its initial temperature. She then added excess zinc powder to the solution and stirred it using a stirrer. She noted down the following data:
Initial temperature 20.5°C
Final temperature 30.0°C
Density of solution 1.0g/cm3
Specific heat capacity of water 4200J/kg/k- Apart from the temperature rise, state one other observation made while she was stirring. (1mark)
- Calculate the heat change for the reaction above (1mark)
- Determine the number of moles of ions of copper reacting (1mark)
- Hence, determine the molar enthalpy of the reaction. (1mark)
- State the name given to :
-
-
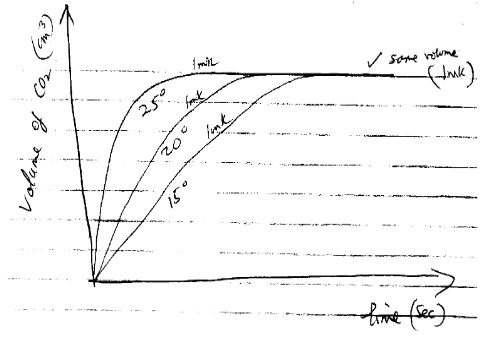
- An experiment was set up to investigate the effect of temperature on the rate of reaction between 1.0g calcium carbonate and excess hydrochloric acid. The temperature was varied from15°C, 20°C and 25°C; and data obtained for the 3 sets of reagents.
- Sketch a graph of volume of carbon (IV) oxide gas produced against time for each temperature on the axes below. Label each graph with corresponding temperature. Consider all gas volumes measured at same temperature and pressure. (2 marks)
- Explain the shape of graphs you’ve drawn in (a) above. (2marks)
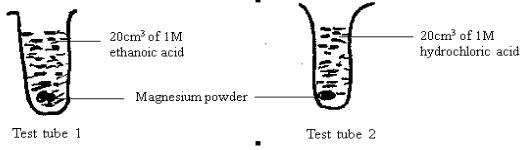
- In an experiment, equal amounts of magnesium powder were placed into test tube 1 and 2 as shown below.
- Explain why the magnesium powder in test tube 2 gets used up faster than that in test tube 1. (3 marks)
- Other than concentration, state one factor that affects the rate of a reaction. (1 mark)
- Consider the equilibrium of the reaction below
A(g) + B(g) ⇌ D(I) + E(g); DH = -ve.
In which direction will the equilibrium position shift as a result of each of the following changes? Explain.- Raising the temperature (1 mark)
- Reducing the volume of the container. (1mark)
- An experiment was set up to investigate the effect of temperature on the rate of reaction between 1.0g calcium carbonate and excess hydrochloric acid. The temperature was varied from15°C, 20°C and 25°C; and data obtained for the 3 sets of reagents.
-
- Work out the oxidation number of nitrogen in NO3- ? (1 mark)
- Study the standard electrode potentials below and answer the questions that follow. (The letters do not represent the actual symbols of the elements.
A+(aq) + e- A(s) E=-2.92V
B+(aq) + e- B(s) E=+0.52V
C+(aq) + e- 12C2(g) E=0.00V
12D2(g) + e- D-(aq) E=+1.36V
E²+(aq) + 2e- E(s) E=-0.44V- With reasons, identify the;
- Strongest reducing agent. (1 mark)
- the reference electrode. (1 mark)
- Write the overall equation for the reaction that will be obtained when half cells of B and E are connected. (1 mark)
- Explain whether the reaction represented below can take place. (2 marks)
2A+(aq)+E(s) 2A(s)+E2+(aq) - Draw the cell diagram obtained when the half cells in (ii) are combined. (2 marks)
- With reasons, identify the;
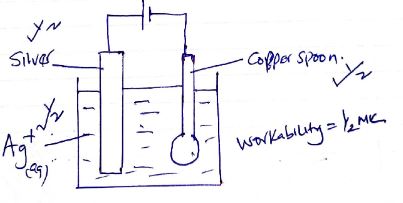
- In an experiment to electroplate a copper spoon with silver, a current of 0.5A was passed for 18 minutes.
- Sketch a diagram to show how the experiment was carried out. (2 marks)
- Calculate the amount of silver deposited on the spoon.
(IF = 96500C, Ag = 108) (2 marks)
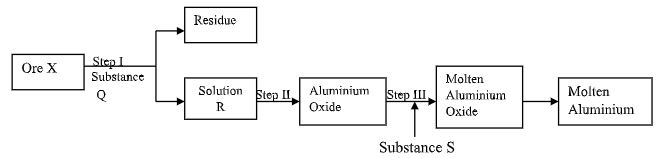
- The process of extraction of Aluminium is summarized as below:
-
- Write the formula of the main Ore X which is used in extraction of Aluminium. (1 mark)
- Name:
- The main residue formed after filtration in step I. (1 mark)
- Substance Q. (1 mark)
- How is the sodium Aluminate in Solution R separated from the impurity silicon (iv) oxide. (2 marks)
- What is the purpose of addition of substance S in step III. Explain. (2 marks)
-
- Explain why the Anode in extraction of Aluminium is replaced periodically. (2 marks)
- Write an equation for the formation of Aluminium at the cathode. (1 mark)
- Explain why Duralum an alloy of Aluminium is used in construction of aircraft parts and car window frames. (1 mark)
-
-
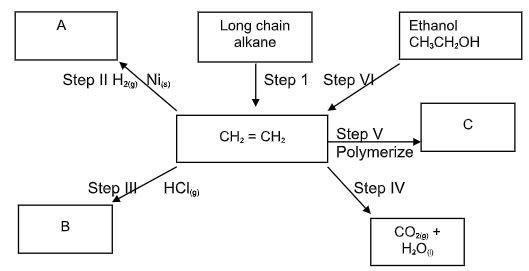
- Study the flow chart below and answer the questions that follow.
- Name the process taking place in step (I). (1mark)
- Describe a chemical test that can be carried out to show the identity of organic compound A. (2marks)
- Give the name of the following: (2marks)
- A:…………………………………………………………………………………
- B:…………………………………………………………………………………
- Give the structural formulae of substance C. (1mark)
- Name the type of reaction that occurs in:
- Step IV (2marks)
- Step VI:
- Give the reagent and the condition necessary for step VI. (2marks)
Reagent:…………………………………………………………………………………………
Condition:……………………………………………………………………………………
- Give the systematic names of the following compounds:
- CH2CHCHCH2CH3 (1mark)
- CH C CH3 (1mark)
- Study the flow chart below and answer the questions that follow.
Marking Scheme
-
-
- Atomic number increases ; number of protons increase
- Atomic radius decreases / reduces; due to the additional protons // increase in nuclear charge hence energy levels are pulled closer to the nuclear
-
-
- 2.8
- 2.8.4
-
- P/Phosphorous ; cut off air// Prevent reaction with air// smoulders when exposed to air
- Manufacture of aluminium sheets //aircraft parts// Aluminium foil// Reject manufacture of Iron sheets.
- White fumes are formed. The Chloride of phosphorous hydrolyses in air to form hydrogen chloride
-
-
-
- Is water that boils above boiling point.
It is achieved by raising pressure of water - To melt the sulphur.
Boiling water is at 100°C while sulphur melts at 113°C - Monoclinic sulphur (stable at temperatures above 96°C)
- Plastic sulphur
- Is water that boils above boiling point.
-
- Electrode that serves only as a source or sink for electrons without playing a chemical role in the electrode reaction
- To increase the surface area for dissolving of the gas.
-
- The pH lowers as SO2(g) formed dissolves in water forming H2SO3 which is acidic
- pH lowers as concentration of H+ increases due to deposition of cu2+ leaving H+ ions in solution
-
- 4OH-(aq) → 2H2O(l) + O2(g) + 4e-
- 4H+(aq) + 4e- → 2H2(g)
-
-
-
- It is the energy released or absorbed when one mole of a compound is formed from its constituent elements in their standard states
-
∆Hf = [2(-394) + 3(-286)- (1560)] = -86kJ/mole-
- ∆H1 Enthalpy of lattice for NaCl(s) (1mark)
- ∆H2 Enthalpy of hydration of Na+(g) (1mark)
-
- ∆H4 = ∆H1 + ∆H2 + ∆H3
∆H4 = +776 + (-402 + -371) = +3 kJ/mole -
- - Colour of solution changes from blue to colourless
- A brown solid was deposited , etc - ∆H = m x c x ∆T
∆H = 100.01000.0 cm3 x 1.0 gcm3 x 4200JkgK x (30.0 - 20.5) K
∆H = -3990J - Number of moles = o.1mole x 100.0 dm31000.0 dm3 = 0.01 m0les
- ∆Hmolar = 1 mole x 3990 J0.01mole = -399 kJ/mole
- - Colour of solution changes from blue to colourless
-
-
-
-
-
- Total volume of gas evolved is equal when excess acid was reacted with same mass of calcium carbonate – graph levels off in the end. (1mark)
The gradient of graph for 25°C in greater showing greater rate of reaction at higher temperature. Increase in temperature increases the kinetic energy of particles causing more frequent and effective collisions w.t.t.e (1mark)
-
-
- Hydrochloric acid completely dissociated in water producing a large amount of H+ whereas ethanoic acid only partially dissociates in water releasing few H+ ions. In ethanoic acid solution there are few H+ available for displacement. W.t.t.e. (3marks)
- Temperature, pressure, surface area, catalyst, etc for any one – 1mark
-
- left/ backward
A(g) + B (g) ⇌ D(I) + E(g) + heat
Increase in temperature means heat which is a product in this case. Equilibrium shift backwards to get rid of excess heat. - Right / forward
The products formed occupy a smaller volume compared to reactants.
- left/ backward
-
-
- X+-2(3)=-1
X=+6-1
X=+5 -
-
- A(s). can easily lose electrons/ most electropositive/most negative electrode potential.
- C2. Has electrode potential of 0.00V
- E(s) +2B+ → 2B(s) +E2+ E= +0.96V
- Reaction cannot take place. A is more reactive than E/ E cannot displace A.
Or has negative EMF. -
-
-
-
- 0.5× 18× 60=540C
96500C=108g
(108×540)÷ 96500= 0.644g
-
- X+-2(3)=-1
-
-
- Al2O3. 2H2O√1 // Al2O3.H2O
-
- Iron (iii) Oxide√1
- Concentrated Sodium√1 Hydroxide // (NaOH)
- By bubbling carbon (iv) oxide gas through the filtrate to precipitate
Aluminum hydroxide which is the filtered off. √½ - To lower M.p of Al2O3 from 2015°C√1 to 800°C; which is economical√1 during electrolysis // to avoid Aluminium from vaporizing if electrolysis is carried out at 2015°C.
-
- Because the carbon anode is attacked√1 by oxygen liberated at high temperature hence the anode gradually burns√1 away.
- 4Al3+(l) + 12e- → 4Al(s) √1
OR 4Al3+(l) + 3e- → Al(s)
-
- It is light, √1 hard, strong and resistant to corrosion.
- -making cooking vessels. √1
- Making overhead cables. √1
- As a reducing agent in thermite process. (any 2x1 = 2mks)
-
-
-
- Cracking√ 1
- When the gas is burnt in air√ 1 it burns with a pale blue flame. √ 1
OR
Does not decolourize √ 1purple acidified potassium manganate (VII). √ 1 -
- A. Ethane√ 1
- B 1- Chloroethane√ 1
-
-
- Combustion√ 1
- Dehydration√ 1
- Conc. H2SO4√ 1
Temperature of 1700C. √ 1
-
- Pent-2-ene√ 1
- Prop-1-yne
-
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 2 Questions and Answers - Mathioya Mock Exams 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students