QUESTIONS
- A given element E has atomic number 14 and consist of isotopes as shown below:
Isotope X Y Z
Isotopic mass 28 29 30
Percentage abundance 92.2 4.7 3.1- What are isotopes? (1mk)
- Determine the relative atomic mass of E. (2mks)
- Passing a small quantity of carbon (iv) oxide through limewater, forms a white precipitate which dissolves when excess carbon (IV) oxide is bubbled through.
- Name the white precipitate. ( ½ mk)
- Explain using a chemical equation why the white precipitate dissolves in excess carbon (IV) oxide. (1 ½ mks)
- What will happen when solution in (b) above is boiled? (1mk)
- Study the set up below and answer the questions that follow.
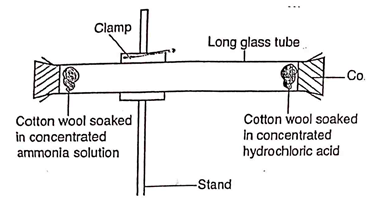
- State and explain the observation made in the tube. (2mks)
- Indicate with a cross (x) on the diagram the likely position where the observation stated in (a) above would be made. (1mk)
- Explain the observation made above in relation to Graham’s law. (1mk)
- In an experiment a small amount of charcoal was added into a test tube and 5cm3 of concentrated nitric (V) acid added, then warmed.
- State the observation that was made. (1mk)
- Explain the observation made in (a) above. (1mk)
- Write an equation for the reaction that took place. (1mk)
- Compare the electrical conductivity of dilute ethanoic acid and dilute sulphuric (VI) acid, explain your answer. (2mks)
-
- What is meant by solubility? (1mk)
- In an experiment to determine the solubility of solid Y in water at 30oC the following results were obtained.
Mass of evaporating dish = 26.2g
Mass of evaporating dish + saturated solution = 42.4g
Mass of evaporating dish + dry solid y = 30.4g
Using the information, determine the solubility of solid y at 30oC in grammes per 100g of water. (2mks)
- The scheme below represents some reactions starting with a white solid A.
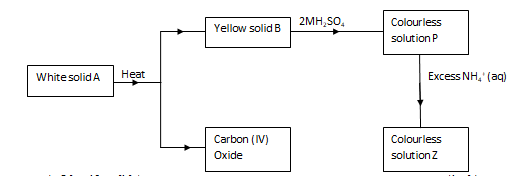
- Identify solid A (1mk)
- Write an equation for the reaction between solid B and 2M sulphuric (VI) acid. (1mk)
- Write ionic equation for the formation of colourless solution Z. (1mk)
-
- Element A and B have atomic numbers 6 and 1 respectively. Using a dot (.) and cross (x) diagram illustrate the type of bond formed when the two elements combine. (1mk)
- Explain why solid sodium chloride does not conduct electricity while sodium chloride solution conducts. (2mks)
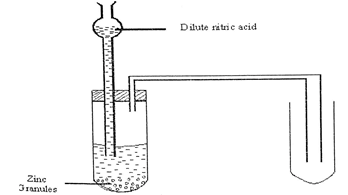
- The diagram below shows the laboratory preparation of hydrochloric acid.
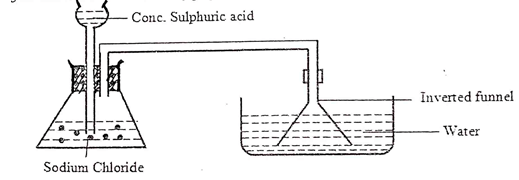
- State the condition necessary for the reaction to occur. (1mk)
- Write a chemical equation for the reaction between sodium chloride and concentrated sulphuric (VI) acid. (1mk)
- Give one reason why an inverted funnel is used instead of delivery tube. (1mk)
- Use the reaction scheme below to and the questions that follow.
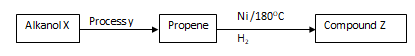
- Draw the structure of alkanol X. (1mk)
- Name process Y. (1mk)
- Write the molecular formula of the 5th member in which propene belong. (1mk)
- The structural formula of compounds Q and R are as follows:
Q CH3CH2CH2COOCH2CH3
R CH3CH2CHCH2CH3
I
HO-C=O- Give the empirical formula that represents both compounds Q and R. (1mk)
- Which single chemical term best describes the two substances Q and R? (1mk)
- Which unique physical property of substance Q is used to identify it? (1mk)
- 18.7cm3 of a dibasic acid, H2A required 25cmv 0.1M sodium hydroxide for complete neutralization.
- How many moles of sodium hydroxide are contained in 25cm3? (1mk)
- Calculate the molarity of the dibasic acid, H2A. (2mks)
- Fill the empty spaces in the table below. (2mks)
Apparatus Use Pipe-clay triangle Pair of tongs - Some reaction of metals P,Q,R, and S are given below.
Metal Reaction with water Reaction with dilute hydrochloric acid P A few bubbles form slowly in water Vigorous reaction. Gas is given off Q Vigorous reaction , metal melts gas given off Explosive reaction (Should not be attempted) R No reaction No reaction S Does not react with cold water. Hot metal reacts with steam Steady fizzing - Arrange the metals in order of the reactivity starting with the least reactive.(1mk)
- Write a chemical equation for the reaction between metal Q and water. (1mk)
- Which of the metals could be copper? Explain. (2mks)
- Below is a set up used to collect hydrogen gas?
- Identify with reason, two mistakes in the set up. (2mks)
- Explain the role of hydrogen in the manufacture of margarine. (1mk)
-
- Identify the acid and base in the above equation using Bronsted Lowry theory. (2mks)
- What causes permanent water hardness? (1mk)
- Study the information in the table below and answer the questions that follow
Element Electron arrangement 1st ionization energy Beryllium 2.2 900 Magnesium 2.8.2 740 Calcium 2.8.8.2 590 - What chemical family does the elements belong to? (1mk)
- What is meant by the term ionization energy? (1mk)
- Explain the trend in the 1st ionization energy of the elements above. (2mks)
- State the role of the following parts during fractional distillation of a mixture of water and ethanol.
- Fractionating column (1mk)
- Glass beads in the fractionating column. (1mk)
- Draw a labeled diagram of the set up of apparatus that can be used to electrolyse lead (II) bromide. (3mks)
- The following table shows pH values of solution of compounds D, E,F and G.
Compound D E F G pH value of solution 2 5 7 13 - State which one of the compounds is likely to be;
- Sodium chloride (1mk)
- Sodium hydroxide. Explain. (1mk)
- Select two compounds that can be used to illustrate the amphoteric nature of an oxide. (1mk)
- State which one of the compounds is likely to be;
- An experiment was carried out to determine the presence of substance P,Q, R and S in the mixture T. The results obtained one shown below
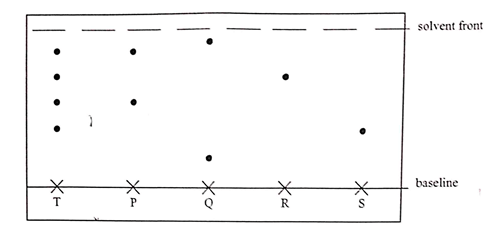
- Name the method of separation illustrated above. (1mk)
- Select;
- One substance which contains a component not present in T. (1mk)
- A substance which is least soluble in the solvent used. (1mk)
- During laboratory preparation of oxygen, manganese (IV) oxide is added to reagent H.
- Name reagent H (1mk)
- State the role of manganese (IV) oxide. (1mk)
- Write the equation for the reaction that takes place. (1mk)
- The heat of solution and hydration energy of potassium chloride is -17.2kJ and -689kJ respectively. Calculate the lattice energy of potassium chloride.(2mks)
-
- Define molar heat of displacement. (1mk)
- The following ionic equation represents the reaction between metal Y and an aqueous solution of Z2+.
Y(s) +Z2+(aq) → Y2+(aq) +Z(s)
ΔH=-ve
Draw an energy level diagram to represent the reaction. (2mks)
-
- What is meant by the term bleaching? (1mk)
- Write the formula of the bleaching agent formed when chloride gas reacts with aqueous sodium hydroxide. (1mk)
- State the role of chlorine in water treatment. (1mk)
- The flow chart below shows the process of obtaining a sample of nitrogen gas. Study it and answer the questions that follow.
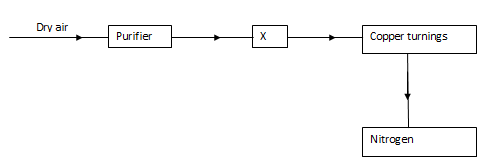
- Identify X (1mk)
- Write an equation for the reaction with heated copper turnings. (1mk)
- Name an impurity in the sample of nitrogen gas. (1mk)
- A mixture contains ammonium chloride, copper (II) oxide and sodium chloride. Describe how each of the substances can be obtained from the mixture. (3mks)

MARKING SCHEME
-
- Isotopes are atoms with same atomic numbers (number of protons ) but different mass numbers (or number of neutrans)
- = (92.2 x 28) + (4.7 x 29) + (3.2 x 30)
100
= 2581.6 + 136.3 + 93.0
100
= 2810.9
100
=28.109
-
- Calcium carbonate/COCO3
- The white precipitate dissolves due to formation of calcium hydrogen carbonate.
CaCO3(s) + CO2(g)+H2O(i) Ca(HCO3)2(aq) - Write precipitate reappears.
Ca(HCO3)2 CaCO3(s)+ CO2(g) + CO2(s)+ H2O(l)
-
- A white solid/ ring was formed inside the combustion tube closer to the cotton wool in concentrated hydrochloric acid, ammonia is lighter and diffuse faster.
- Gases with low densities diffuses faster than those with high densities.
-
- Brown fumes of nitrogen (IV) Oxide
- Nitric (V) acid is a strong oxidising agent and thus oxides carbon to carbon to carbon (IV) oxide and itself reduced to nitrogen (IV) oxide.
- C(s) + 4HNO3(l) 4NO2(g)+ CO2(g)+ 2H2O(l)
- Dilute sulphuric (VI) acid has a higher electrical conductivity than dilute ethanoic acid; dilute H2SO4 acid has more hydrogen ions in solution, being a strong acid than dilute CH3COOH acid.
-
- Solubility is the maximum amount of solute that will dissolve in 100g of water at a given temperature or
Is the mass in grammes of solute required to saturate 100g of water at a given temperature. - Mass of solid Y = (30.4 – 26.2) = 4.2g
Mass of water in the solution = (42.4 -30.4)
=12g
12g of water dissolves 4.2g
Thus 100g of water dissolves =?
= 100 x 4.2
12
= 35g/100g H2O
- Solubility is the maximum amount of solute that will dissolve in 100g of water at a given temperature or
-
- Zinc carbonate
- ZnO(s) + H2SO4(aq) → ZnSO4(aq) + H2O(l)
- Zn(OH)2(s) + 4NH3(aq) → [Zn(NH3)4]2+(aq)+ 2OH(aq)
-
- A = 2.4
B=1 - Solid sodium chloride does not contain free ions while sodium chloride solution contains free ions.
- A = 2.4
-
- Heating
- NaCl(s) +H2SO4(l) → NaHSO4(aq) + HCl(g)
- Provides larger surface area over which dissolving of gas takes place.
Prevents suck back of solution which causes breakage of the hot apparatus.
-
-
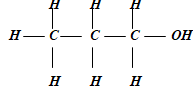
- Dehydration
- C6H12
-
-
- C6H12O2
- Isomers
- Pleasant / sweet/ fruity smell
-
- 1000cm3 → 0.1moles
25cm3 = ?
=25 x 0.1
1000
=0.0025 moles - H2A(g) + 2NaOH(aq) → Na2A(aq) + H2O(l)
Mole ratio = 1:2
Moles of acid = 0.0025
2
= 0.00125 moles
Molarity = 1000 x 0.00125
18.7
= 0.0668M
- 1000cm3 → 0.1moles
Apparatus Use Pipe-clay triangle Used for supporting crucibles during heating Pair of tongs -
- R, S, P, Q → Increasing reactivity
- 2Q(s) +2H2O(l) → 2QOH(aq) + H2O(l)
- R; copper doesn’t react with both dilute acids and water since it is lower than hydrogen in reactivity series.
-
- Hydrogen gas cannot be collected by downward delivery as it is lighter than air.
No gas collected as dilute nitric (V) acid would oxidize it to water. - Hydrogen breaks the double bonds in liquid oil making it saturated thus solidifies.
- Hydrogen gas cannot be collected by downward delivery as it is lighter than air.
-
- Acid
Base
Reason; H2O donates H+ ions to NH3 in the reaction. - Presence of MgSO4 and CaSO4
- Acid
-
- Alkaline earth metals
- The energy required to remove an electron from an atom in gaseous state.
- Ionization energy decreases down the group due to increase in atomic radii down the group which reduces the attraction of the elecetrons in the outermost energy level towards the nucleus hence ease of loss of electrons from the outermost energy level.
-
- It allows water vapour to condense into liquid and flow back into the flask before the boiling point of water is reached.
- To increase the surface area for condensation process.
-
-
- F
- G; NaOH is a strong alkali with high pH
- D and G
-
-
- Paper chromatography / chromatography
-
- Q
- S
-
- Hydrogen peroxide
- It is a catalyst
- H2O2(l) → 2H2O(l) + O2(g)
- ΔH solution = ΔH lactice + ΔH hydration
ΔH latt = -17.2 – ( - 689)
=+671.8KJ - The heat revolved / the enthalpy change when one mole of a substance is displaced from its ions in solution.
-
- removal of original colour/decolourising
- BaClO or NaOCl
- Kills micro organism / germs / bacteria
-
- KOH/NaOH
- 2Cu(s) + O2(g) → 2CUO(s)
- Argon, neon; inert gases/noble gases
-
- Put the mixture in the beaker
- Place the beaker on a tripoid stand, cover it with a watch glass with cold water.
- Heat the mixture to sublime the NH4Cl, collect it as sublimate on cooler surface of watch glass.
- Add water to the remaining mixture. NaCl dissolve filter to obtain copper (II) Oxide.
- Evaporate the filtrate to concentrate the NaCl. Allow the hot solution to cool and turn crystal.
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 1 Questions and Answers - Londiani Joint Mock Exams 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students
