QUESTIONS
- The table below shows the positions of some elements in the periodic table. The letters are not the actual symbols of the elements.
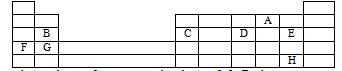
- Select an element that can form an ion with a charge of +2. Explain your answer (2mks)
- What type of structure would the oxide of C have? Explain your answer. (2mks)
- How does the reactivity of H compare with that of E? Explain your answer.(2mks)
- Explain how you would expect the following to compare.
- Atomic radii of F and G.(1mk)
- The pH values of aqueous solution of the oxide of B and D.(1mk)
- Draw a diagram to represent an ion of element D. (2mks)
- The diagram below shows a set up for the determination of enthalpy of displacement for the reaction between zinc metal and copper (II) sulphate solution
- Define molar heat of displacement (1 mark)
- Write an ionic equation for the reaction that takes place in this experiment(1 mark)
- What is the function of the saw dust in the set up (1 mark)
- State and explain two observations made at the end of this experiment other than rise in temperature (3 marks)
- 4 g of the zinc powder were added to 50cm3 of 0.25M copper (II) sulphate solution. The mixture was stirred with the thermometer and the highest temperature recorded.
Final temperature = 34.5ºC
Initial temperature = 22.0ºC
Calculate the molar heat of displacement of copper by zinc (Zn=65) (4marks) - Sketch an energy level diagram for the above reaction (2 marks)
-
- Study the given reduction potentials and answer the questions that follow. The letters do not represent actual symbols of elements.
Eθ(V)
X2+(aq) + 2e- → X(s) -2.90
Y2+(aq) + 2e- → Y(s) -2.38
Z2+(aq) + 2e- → Z(s) 0.00
½ A2(g) + e- → A(aq) +2.87- Which element is likely to be hydrogen? (1mk)
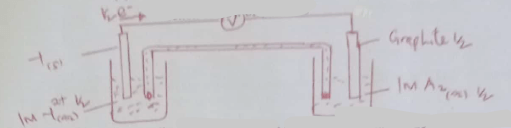
- Draw an electrochemical cell when Y and A are combined. Show the direction of flow of electrons (2mks)
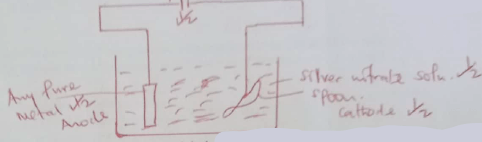
- Draw a diagram to show how a spoon made of iron can be coated with silver metal (2mks)
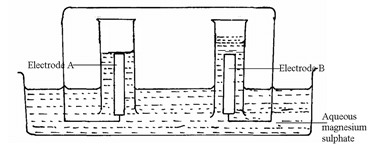
- The set – up below was used during the electrolysis of a solution of magnesium sulphate using inert electrodes.

- Identify the ions present in the electrolyte (1mk)
- Write half equations at the anode and at the cathode:
Cathode: (1mk)
Anode (1mk) - Which electrode is the cathode? Explain (2mks)
- Explain the pH changes of the electrolyte during the experiment (2mks)
- Calculate the quantity of electricity (in coulombs) that would liberate 1.2dm3 of oxygen gas at R.T.P (take 1 mole of gas at r.t.p = 24dm3, 1F = 9650ºC) (2mks)
- Study the given reduction potentials and answer the questions that follow. The letters do not represent actual symbols of elements.
-
- Crude oil is a source of many compounds that contain carbon and hydrogen only.
- Name the process used to separate the components of crude oil. (1mk)
- On what basis does separation occur (1mk)
-
- Under certain conditions, hexane can be converted to two products, one of them being butane.
Write the formula of the other product (1mk) - Describe a simple chemical test to show the differences between the two products formed in (b) above. (2mks)
- Under certain conditions, hexane can be converted to two products, one of them being butane.
- Ethyne is another compound found in crude oil. One mole of hydrogen chloride gas reacted with one mole of ethyne and a product P was formed. P was then reacted with excess hydrogen gas to form product Q.
Draw the structure of P and Q (2mks) - Ethyne may be collected over water during preparation. Explain why this is possible. (1mk)
-
- When one mole of ethyne is reacted with one mole of hydrogen, the product formed may undergo addition polymerization under suitable conditions. Write an equation for addition polymerization of the product. (1mk)
- Give one disadvantage of the polymer in e(i) above (1mk)
- State one commercial use of ethyne (1mk)
- Crude oil is a source of many compounds that contain carbon and hydrogen only.
- In the experiment, steam was passed over heated iron wool as shown in the diagram below.The gas produced was then dried and passed through heated copper (ii) oxide
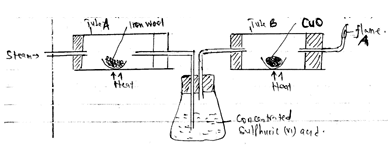
- Write an equation for the reaction between steam and iron 1mk
- What observation would be made in tube B at the end of reaction? Explain 2mks
- What precaution should be taken into consideration before lighting the gas at A 1mk
- What type of reaction takes place in the tube B 1mk
- Give two uses that are for both carbon(ii) oxide and hydrogen gases 2mks
-
- Give the name of the process described below 3mks
Substance Condition Name of the process Iron(ii) sulphate heptahydrate Exposed to air ,changes from crystalline to powder form Concentrated sulphuric(vi) acid Exposd to air, volume of the acid increases Zinc nitrate Exposed to air changes in solution - Name another substance that can undergo the same process as zinc nitrate above 1mk
- Write the formula of copper (ii) sulphate crystals 1mk
- Give the name of the process described below 3mks
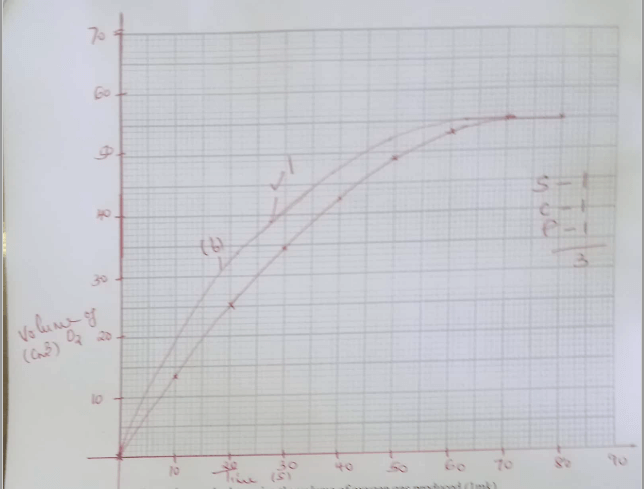
- 3.06g of Manganese (IV) oxide was placed in a flask and 25cm3 of hydrogen peroxide added.
The volume of oxygen gas produced was recorded after every 10 seconds. The results obtained were recorded in the table below.Time(s) 0 10 20 30 40 50 60 70 80 Volume(cm3) 0 13.5 25 34.5 42.5 49 53 55 55
Plot a graph of volume (cm3) against time (sec). (3mks)- From the graph, determine the volume of oxygen gas produced.(1mk)
- The experiment was repeated using more concentrated hydrogen peroxide.
On the same axis; sketch the curve that was obtained. (1mk) - Write an equation for catalytic decomposition of hydrogen peroxide.(1mk)
- Give the test for oxygen gas.(1 mk)
- State two commercial uses of oxygen gas. (2 mks)
- The scheme below shows various reactions starting with ammonia. Study it and answer the questions that follow.
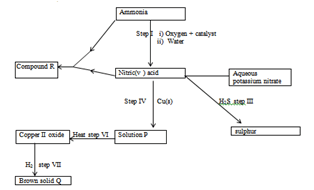
- Name:
- Compound R (1mk)
- Solid Q (1mk)
- Catalyst used in step I (1mk)
- Process taking place in step II (1mk)
-
- What property of nitric (V) acid is demonstrated in step III (1mk)
- State the precaution to be taken when carrying out reaction in step III? Give a reason (2mks)
- Write an equation for the reaction in step VII (1mk)
-
- Give one use of compound R (1mk)
- Calculate the percentage of nitrogen by mass in compound R (N=14, H=1, 0= 16 (2mks)
- State one commercial use of Nitric (V) acid apart from making nitrogenous fertilizers (1mk)
- Name:

MARKING SCHEME
-
- B or G - due to lose if 2 electrons in the outermost energy level.
- Giant ionic structure - this results in farming of ionic bond and builds up to form giant ionic structure.
- E is more reactive than H. E is more electronegative/smaller in size than H/ easily gains an electron than H.
-
- F has a bigger/larger atomic radius than G since G has a stronger nuclear charge attraction/more protons than F.
- PH of B is more than 7 and D is less than 7
- 2.8.5 D3- 2.8.8

Distribution of es
showing the charge
-
- is the enthapy change that occurs when one mole of a substance is displaced from a solution of its ions
- Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
- To prevent heat loss or absorbed by the apparatus
- the blue color of the solution turns to colourless and brown solid is seen - zinc displaces Cu2+ ions from the solution and get defrosted. The plastic beaker becomes warm - due to heat absorbed by the apparatus
- Heat change = Mcθ = ( 50 x 4.2 x 12.5)kj
1000
= 2.625kJ
moles of Cu2+ions = 50 x 0.25
1000
= 0.0125
0.0125moles = 2.625kJ
1 mole = ?
( 1 x 2.625)kJ/mole
0.0125
= 210kJ / mole
= -210kJ/mole -
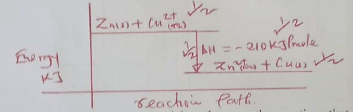
-
-
- Z - it has Eθ = 0.00
-
-
-
- Mg2+, SO42- , H+ , OH-
- Cathode
4H+(aq) + 4e → H2(g)
Anode
4OH-(aq) → O2(g) + 2H2O(I) + 4e - Electrode A from the equation 2 moles of H2:1mole of O2
- Mg2+, SO42- , H+ , OH-
- The pH remained the same since equal amount of OH- and H+ are removed during electrolysis
- 4OH-(aq) → 2H2O + O2 + 4e
4e = 4f = 4 x 96520H2 = 240m3
? = 1.2m3
(4 x 96520 x 1.2)C
24
= 19.300C
-
-
-
- Fractional distillation
- Miscibility of the components/fractions
close range of boiling points
-
-
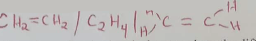
- Pass the two gases separately through acidified, KMNO4. Ethene decolorises the purple solution while C4H8 doesn't or through bromine water/acidified K2Cr2O4/ burning the gas (any of them)
-
-
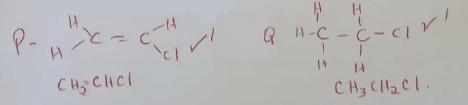
- Insoluble in water
-
-
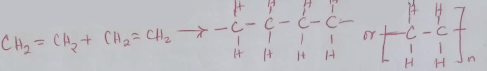
- non-biodegradable/ does not rot easily
-
-
- oxy-acetylene flame for welding
- manufacture of synthetic fibres eg rayons
- manufacture of PVC
-
-
- 3Fe(s) + 4H2O(g) → FeO4(s) + 4H2(g)
- Black solid turns brown / H2 reduces CuO to Cu metal
- Excess H2 should be passed before lighting it to avoid explosion
- Reduction
- Both are reducing agents of metallic oxides
Both are used as fuels -
-
Substance Condition Name of the process Iron(ii) sulphate heptahydrate Exposed to air ,changes from crystalline to powder form Effervesvence Concentrated sulphuric(vi) acid Exposd to air, volume of the acid increases Hydroscopy Zinc nitrate Exposed to air changes in solution Deliquescence - Sodium hydroxide, potassium hydroxide , Iron(II) chloride/Iron(III) chloride
- CuSO4 . 5H2O
-
-
- 55cm3 (must be shown on the graph)
- 2H2O2(I) MnO2 2H2O(I) + O2(g)
- relights/reignites/rekindles a glowing splint
-
- in hospital to patients with breathing problems
- oxyacetylene flames in welding
- in mountain climbinbg
- in sea diving
-
-
-
- Ammonium nitrate
- copper
- platinum - rhodium/platinum
- neutralization
-
- oxidizing property
- carry out in fume chamber / in the open air H2S is poisonous
- CuO(s) + H2(g) → Cu(s) + H2O(I)
-
- As a fertilizer/making of explosives
- NH4NO3 = 80
%N = 28/80 x 100 = 35%
-
- Making synthetic fibres
- Making aqua regia
- Used in extraction of gold
- Making explosives TNT(any one)
-
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 2 Questions and Answers - Londiani Joint Mock Exams 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students
