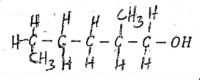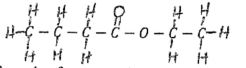Questions
Instructions to Candidates
- Answer all the questions in the spaces provided
- Scientific calculators may be used
- All working must be clearly shown where necessary
-
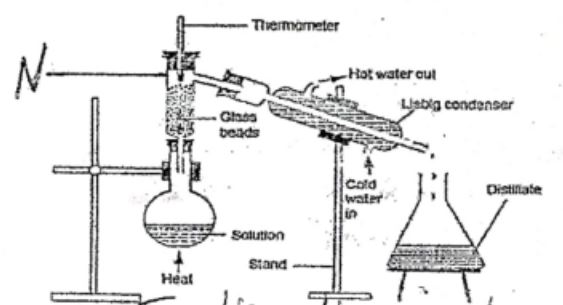
- Explain the term 'strike back' as applied to a Bunsen burner (1mk)
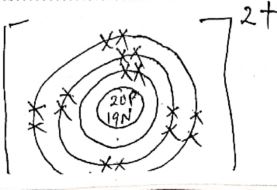
- Give the name and state the function of the apparatus labeled N in the diagram shown below (2mks)
Name
Function
- 1.6g of Ammonium nitrate were dissolved in 100cm3 of water at room temeprature 21°C and the mixture was stirred wiht a thermometer. The molar heat of solution obtained in the experiment was +126Kj/mol, calculate the final temperature of solution (3 mks)
(C = 4.2kJ/Kg/K, Density of solution 1g/cm3, N=14, H=1, O= 16) - Describe how constant mass of copper can be determined in copper II carbonate (3 mks)
-
- Define the term Homologous series (1mk)
- Hydrocarbon A with 3 carbon atoms decolorizes bromine water in the presence of light but does not decolorize acidified Potassium Manganate VII
- Name the homologous series to which hydrocarbon A belongs (1 mk)
- Write the chemical equation to show how the Hydrocarbon A s prepared in the laboratory. (1mk)
-
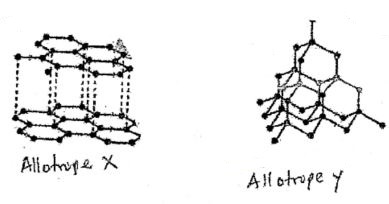
- The diagram below shows allotropes of Carbon. Study them and asnwer questions that follows
State- One use of allotrope X
- Why allotrope Y is very hard
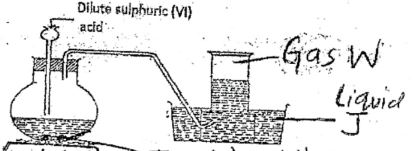
- Set up below is used to prepare gas W
- Identify gas W and liquid J (1 mk)
Gas W
Liquid J - State the observation made when gas W is bubbled in lead (II) nitrate solution (1 mk)
- Identify gas W and liquid J (1 mk)
- The diagram below shows allotropes of Carbon. Study them and asnwer questions that follows
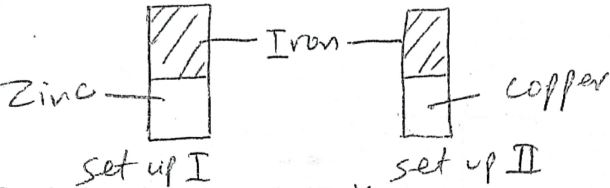
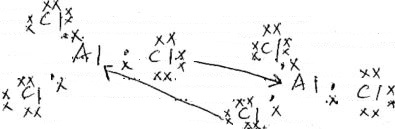
- A form two student in an attempt to stop rusting placed copper and zinc metals in contact with iron separately as shown below.
- State the observation made set up I and II (1 mk)
Set I
Set II - Explain your answer in (a) above (1mk)
- Name the method of preventing rusting illustrated above (1 mk)
- State the observation made set up I and II (1 mk)
-
- State Graham's law of diffusion
- 100cm3 of Carbon (IV) oxide gas diffused through a porous partition in 30 seconds, How long would it take 150cm3 of Nitrogen (IV) oxed to diffuse through the same partition under similar condiitons. C= 12, N= 14, O= 16
- An element M has 19 neutrons and a mass number of 39
- Write the electron arrangement of its stabel ion (1mk)
- Which period does M belong to (1 mk)
- Draw the structure of its ion (1mk)
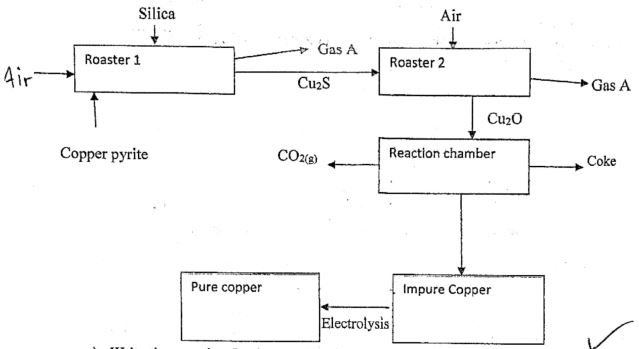
- The flow chart below shows stages in extraction of copper , use it to answer questions that follow
- Write the eqaution for the reaction that occurs in the roaster (1 mk)
- Name Gas A (1 mk)
- What is the importance of assing silica in roaster 1(1 mk)
-
- Using dots (.) and crosses (x) to represent electrons draw the structure of aluminium chloride dimer
- Explain why aluminium carbonate does not exist.
- Melting point of lithium chloride is higher than sodium chloride.
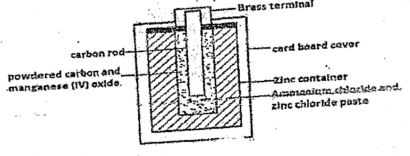
- The diagram below is a cross section o the dry cell. Study it and answer questions that follow.
- Write the overall equation to represent the reaction that takes place in the cell.
- The carbon rod is surrounded with a mixture of powdered carbon and manganese IV oxide.
What is the function of Manganese IV oxide (1mk) - Explain why a-brass CAp is suitable over copper cap in the above cell. (1 mk)
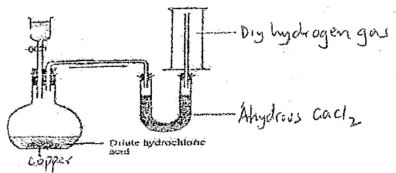
- The diagram below shows preparations of Hydrgen gas. Study it and answer questions that follow.
- Explain why no hydrogen was produced.
- When the mistake is corrected, hydrogen gas is produced name the method used to collect the gas and give reason why it was used.
Method
Reason - Describe a test that can be done to identify Hydrogen gas in the laboratory
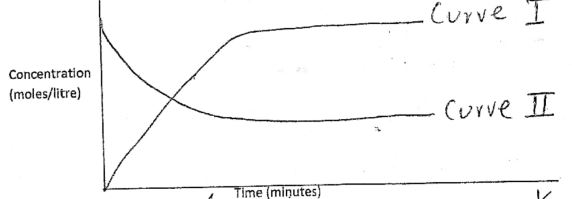
- The equation and the curves below shows decomposition of dinitrogentetraoxide.
N2O4 ⇌ 2NO2(g) ΔH = +17kJ/mol
pale yellow brown- Which curve represents change in concentration of NItrogen (IV) oxide. Explain. (1½mks)
- State and explain the observation made when the beaker containing the mixture is placed in hot water.
- A student bubbled chloride gas through a solution of Magnesium bromide in a corked conical flask.
- State and explain the observation made.(2mks)
- Write the ionic equation for the reaction which occurered at the conical flask. (1mk)
-
- It is not appropriate to refer to group VIII elements as 'Inert gases'. Explain giving an example. (2mks)
- Give one use of them (1mk)
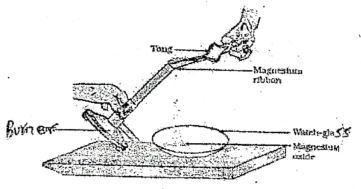
- The diagram below shows burning of Magnesium in air and collecting the products
- Name the observation made during the reaction (1mk)
- Water was added to the product formed, a colourless gass with a pungent irritating smell was produced.
- State the chemical test for the colourless gas (1mk)
- Write the equation leading to fomation of colourless gas (1 mk)
-
- Name the compound below (1mk)
- Compound M reacts with ethanol in the presence of few drops concentrated of sulphuric (VI) acid to form a fruity smelling compound below.
- Write the molecular fromula of compound M (1 mk)
- Give one use of the fruity smelling compound (1 mk)
- Name the compound below (1mk)
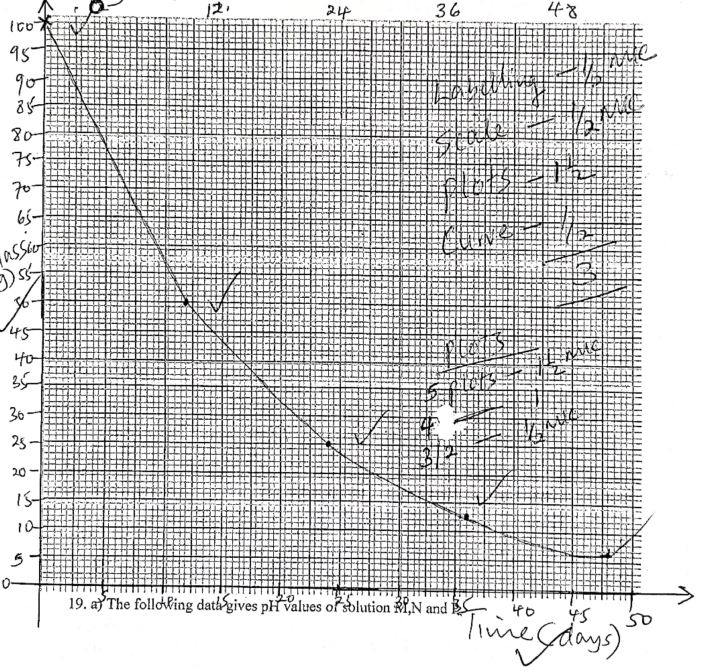
- 100g of 23191Th with a half life of 12 days decayed to a mass of 6.25g on the grid below, plot the graph of 23191Th against time (3mks)
-
- The following data gives pH values of solution M, N and P
Solution pH value M
N
P13.6
7.0
1.3- Which solution will produce carbon iv oxide when reacted with copper (ii) carbonate (1mk)
- What would be the colour of solution M after adding a few drops of phenophtalein (1 mk)
- The graph below shows how the pH value of soil in a farm over a period of time.
State one factor that may have been responsible for the change in soil pH in the interval AB(1mk)
- The following data gives pH values of solution M, N and P
- When 80cm3 of Hydrogen gas were mixed with 60cm3 of chlorine and mixture exploded in a bright sunlight. Reaction took place according to the equation below.
H2(g) + Cl2(g) → 2Hcl(g)- Determine the volume of resulting gas mixture (1mk)
- When the resulting gas mixture was shaken well with water, the volume the gas was found to be less than the original mixture
- Why was the volume reduced (1mk)
- Determine the volume the residue gas after the reduction (1mk)
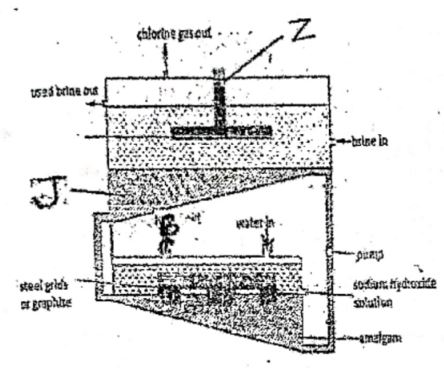
- The diagram below represents the mercury cell used in the industrial manufacture od sodium hydroxide. Study it and answer questions that follow.
- Name the substance B (1mk)
- Write the equation for the reaction that takes places at electrode Z(1 mk)
- Give one reason why electrode J is made up of mercury (1mk)
- Student was required to prepared crystals of Magnesium chloride, starting with 100cm3 of 2M Magnesium Hydroxide. Describe how the student prepared pure dry crystals of magnesium chloride. (3mks)
- In an experiment to separatea mixture xanthophyll and Chlorophyll in plant leaf.
- Describe a procedure that was carried out first before separating the two pigments (2mks)
- After sometime it was discovered that Xanthophyll is more soluble than chlorophyll in the solvent. Draw a well-labelled diagram to show the results of this experiment (1 mk)
- The table below shows the tests carried out on a sample of solution and the results obtained as shown on the table below:
Tests Results I Addition of excess sodium hydroxide White precipitate soluble in excess II Addition of excess aqueous ammonia solution White precipitate soluble in excess III Addition of acidified Barium Nitrate White precipitate. - Identify the Anion in the solution (1mk)
- Write the ionic equation for the reaction in step III (1 mk)
- Write the formula of the complex ion formed in step II (1mk)
- In the laboratory, Nitric V acid can be prepared using the set up below
- Name (1mk)
- Apparatus N
- Reagent R
- Give a reason why sodium nitrate is preferred over other nitrates in above experiment (1mk)
- State one property that makes reagent R suitable for use in this experiment (1mk)
- Name (1mk)
- Potassium carbonate cannot be manufactured by the solvay process. Explain. (1mk)
- The setup below was used to collect dry sample of a gas.
Give two reasons wy the set up above is suitable for collecting Carbon IV oxide(2mks)
Marking Scheme
-
- Phenomenon where the flame goes down the chimney ans goes off. It happens when thegas is being burnt faster than can be supplied.
- Name - Fractionating column
Function - Condenses vapour of a liquid with higher boiling point back to flask before attaining its boiling point.
- Moles of NH4NO3 used
1.6 - 0.02 moles
ΔH = 1 mole → 126 = 2.52Kg
2.52 = 100/1000 x 4.2 x ΔT
ΔT = 6°C
Final temp = 21 - 6
= 15°C -
- Weigh the mass of the crucible and CuCO3
- Heat CuCO3 in a crucible strongly to form CuO as residue
- Pass dry hydrogen over heated CuO to from Cu metal.
- Determine(weight) the mass of the residue.
- Subtract the mass of the crucible from the mass of the residue and crucible.
-
- Sequence of compounds with the same chemical properties, chemical fomula and functional group and they exhibit gradual change in physical properties.
-
- Alkane
- C3: H7COONa(s) + NaOH(s) → C3H8(g) + Na2CO3(s)
(ignore state symbols off ommitted otherwise penalize wrong states)
-
-
- Dry lubricant, positive terminal in dry cell, pencils
-
- Strong covalent bonds between carbon atoms that are uniformly distributed
- Close packaging of the carbon atoms
-
-
- Gas W - hydrogen sulphide/ H2S
Liquid J - warm water
- Gas W - hydrogen sulphide/ H2S
- Black precipitate.
-
-
-
- Set I - Iron remains grey
Set II - A brown coating is formed on iron - In setup I, iron did not rust since zinc offers sacrificial portection
In setup II, itron ruysted since copper is less reactive and offered no protection. - Sacrificial protection.
- Set I - Iron remains grey
-
- Under similar conditions of temperature and pressure , the arte of diffusion of a gas is inversely proportional to the square root of its density.
- 100cm3 of CO2 → 30
150 → ?
150 x 30 = 45
100
TCO₂ = √MCO₄
TNO₂ √MNO₂
45 = √44
TNO₂ √46
TNO₂ = 46.011 seconds
-
- 2.8.8
- Period 4
-
-
- 2CuFeS2(s) + 4O2(g) → Cu2S(s) + 2FeO(s) + 3SO2(g)
- Sulphur (iv) oxide
- Reacts with iron (ii) oxied to form iron (iii) silicate (slag)
-
-
- Aluminium salts hydrolyzes in water to form hydrogen ions which reacts with carbonates to form CO2 gas
- LiCl has stronger ionic bond than NaCl since Li forms small ionic radius than Na.
-
-
- ZnC(s) + 2NH4+(aq) → Zn2+(aq) + 2NH3(g) + H2(g)
- Deplarizes/ oxidizes hydrogen gas to water preventing accumulation of bubble at the positive terminal.
- Brass is resistant to corassion than copper/ it is highly conductive and perfect for electrical parts.
-
- Copper is lower than hydrogen in the reactivityy series hence cannot displace hydrogen nfrom the dilute acid.
- Method - Upward delivery/ downward displacement of air
Reason - hydrogen is less dense than air/ lighter than air. - Introduce a burning splint into a gas jar containing hydrigen gas. It extinguishes the burning splint wiht a 'pop' sound.
-
- Curve I - Increase concentration of NO2
- Brown colour intesnifies - Equilibrium shifts forward since endothermic reaction is favoured by increase in temperature.
-
- Solution changes to brown - Cl2 displaces Br from the solution.
- 2Br -(aq) + Cl2(g) → 2Cl -(aq) + Br2(l)
-
- Some group VIII elements are chemically reactive because of larger atomic radius hence can lose electrons eg xenon.
- Research baloons/ Arch welding
-
- Brilliant white flame
-
- Introduce glass rod dipped in concentrated HCl, white fumes are formed.
- Mg3N2(s) + 6H2O(l) → 3Mg(OH)2(aq) + 2NH3(g)
-
- 2-Methylhexan-1-ol
-
- C3H7COOH/C4H8O2
- Fresheners
Cosmetics
Perfumes
- 100g → 50 → 25 → 12.5 → 6.25
0 12 24 36 48 -
-
- P
- pink
- Acidic rain/ leaching/ water logging
-
-
- H2 : Cl2: 2HCl
1: 1: 2
60: 60 : 120
Volume of H2 used 80 - 60 = 20cm
Total volume = 20cm3 + 120 = 140cm3 -
- HCl(g) dissolved in H2O
- 140cm3 - 120cm3 = 20cm3 of H2(g)
- H2 : Cl2: 2HCl
-
- Hydrogen gas
- 2Cl -(aq) → Cl2(g) + 2e-
- Hg prevents / blocks discharge of H+(aq) at chatode
- Mg(OH)2(aq) + 2HCl(aq) → MgCl2(aq) + 2H2O(l)
1 : 2
100 : 200
- Add 200cm3 of 2MHCl to 1000cm3 of 2MMg(OH)
- Evaporate the resulting solution to saturation.
- Cool the solution
- Dry crsytals betwen filter paper.
-
-
- Crush the leaves in mortar using pestle
- Add propanone and continue crushing
- Decant the solution formed into a beaker.
-
-
-
- SO2-4/ sulphate ion
- Ba2+(aq) + SO2-4(aq) → BaSO4(s)
- (Zn(NH3)4)2+(aq)
-
-
- Retort flask
- Concentrated sulphuric (VI) acid
- Lacks water of crystalization
- Less volatile
-
- KHCO3 is more soluble than NaHCO3 hence does not crsyatllize
- CO2 does not react with H2SO4
CO2 is denser than air
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 1 Questions and Answers - Mokasa II Mock Exams 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students