QUESTIONS
- Briefly describe how chromatography is used to detect illegal steroids used by athletes (3mks)
- State one physical process which is
- Endothermic
- Exothermic
- One large scale source of alkanes is through fractional distillation of crude oil, state one other large scale source in which alkanes can be obtained. (1mk)
-
- State and explain the observation made when chlorine is bubbled through a solution of potassium bromide. (2mks)
- Write the ionic equation for the reaction in 4(1) above.
-
- When element J was placed in water the following observations were made.
- Float on the surface of the water
- Darts on the surface of the water
- Melt to a silvery ball
Give the name given to group in which element belongs
- State one observation made if calcium was used instead of element J.
- When element J was placed in water the following observations were made.
- Study the information below and answer the questions that follow.
Cr2(aq)O2-7 + OH- ⇌ Cr2O2-4 + H2O
Orange Yellow- Which of the two curves below represent the concentration of Cr2O2-7 Explain (2mks)
-
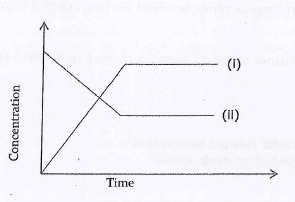
Using the graph above deduce the colour of the solution mixture at equilibrium. - What be observed if dilute hydrochloric acid is added to the reaction mixture in 6 above. Explain
- When a solute is dissolved in a solvent the temperature of the solution will either a drop or a rise. Give a reason why a solute dissolves in solvent may result to a drop in temperature.
- The structure below was formed when a certain hydrocarbon was bubbled through bromine water.
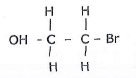
- State the observation made during the process.
- State the homologous series to which the hydrocarbon belongs.
- Draw the structure of the 3rd member of the hydrocarbon above
- A piece of sodium metal was put in propanol.
- State the observation made above.
- Write the equation for the above reaction.
- State and explain the observation made when a red litmus paper was put in the resultant solution.
- State one application of radioactivity in
- Agriculture
- Medicine
-
- State two impurities found together with bauxite.
- Explain how the impurities named above are removed at the purification stage.
- During extraction of aluminium metal the anode is periodically replaced at the electrolysis stage. Give a reason.
- Use the Information provided to answer the questions that follow.
C graphite + O2 CO2 → ΔH-393kJ/mole
C diamond + O2 CO2 → ΔH-395kJ/mole- Determine the enthalpy changes that occur when diamond is formed from graphite
- Is the formation of diamond from graphite endothermic or exothermic?
- Study the diagram below and answer the question that follows.
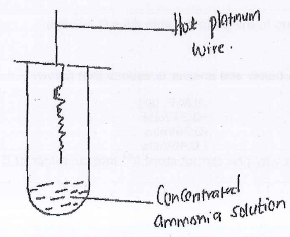
- State two observations made
- Write the chemical equation taking place on the surface of platinum wire.
-
- Write the chemical formula of Trona.
- After extraction of Trona in Lake Magadl. It is taken to the washery in the processing plant. Briefly describe the other processes that follow from the washery till sodium carbonate is obtained.
- An element Q in the periodic table Q not the actual symbol is in period III it forms an oxide with a formula Q203
- Calculate the oxidation number of Q.
- Write the electronic arrangement of A in the above compound. (imk)
- To which group in the periodic table does Q belongs.
- Use the Information below and answer questions that follow.
Element E.M.F. (Eº)
A2++ 2c +0.34Volts
B2+ + 2e -0.76Volts
C2++ 2e -0.46Volts- Give a reason why one cannot store A+ into container of C
- Show if the below reaction can occur or not.
B2+(aq) + C → C2+(aq) and B
- State how each of the following changes.
- pH during electrolysis of copper (II) sulphate using carbon electrodes. (2mks)
- Concentration during electrolysis of sodium sulphate using carbon electrode.
- Write equations to show removal of water hardness by
- Temporary water hardness by boiling
- Addition of sodium carbonate to remove permanent water hardness use ionic equation.
-
- Define the term solubility.
- If saturated solution at 25°C containing 50g saturated solution was heated till all the water evaporated and 10 grams of salt was left. Calculate the solubility of the salt. (2mks)
-
- Study the table below and answer the questions that follow.
If the elements are in the same period arrange them as they occur from left to right.Element Atomic Radius Ionic Radius R
Q
T
S0.436
0.234
0.371
0.1240.123
0.348
0.254
0.671 - Identify
- Metallic elements
- Non-metallic elements
- Identify the metallic element with the highest melting point and give a reason.
- Study the table below and answer the questions that follow.
- State one way in which the purity of a substance can be tested.
-
- Define the term dative bond.
- Using dot(.) and cross(x) show the bonding in hydroxonium ion. (1mk)
- The equations below show the ionization of two acidic substances in water.
H2A(aq) -H2O→ HA-(aq) + H+(aq)
H2B(aq) -H2O→ HB-(aq) + 2H+(aq)- Identify the acid which is a better conductor of electricity and give a reason. (2mks)
- Name the acid that is likely to have a pH value that ranges between (pH4 - PH6)
-
- Define the term acid according to Bronsted lowry theory
- Given the equation
RH3 + H2B ⇌ RH4+ + HB
Which substance is the acid in the forward reaction.
- Write the ionic equation that occurs
- At the negative terminal of a dry cell.
- Negative terminal lead acid accumulator
- The Information below is used in the preparation of a cleansing agent. Study it and answer the question that follows.

- Give the type of the cleansing agent.
- Name substance Y
- Name the type of reaction taking place in step II above.
- Give the main difference between the bleaching of sulphur IV oxide and chlorine.
- Starting with solid aluminlum sulphate describe how a solid sample of aluminium oxide can be prepared.
- Define salt.
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 1 Questions - Mangu High School Mock Exams 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students
