QUESTIONS
-
- The table shows information about some elements (Letters are not the actual symbols of the elements)
Element K M N P Q R S T Atomic number 11 12 13 14 15 16 17 18 - Write the electronic arrangement of the most stable ion of Q. (1 mark)
- Identify the strongest reducing agent. Explain. (2 marks)
- Name the type of structure and bond type present in element P in its pure elemental form. (1 mark)
Structure type : …………………………………………...…………………………………….
Bond type : ……………………………………………………………...……………………… - Give two reasons why the oxide of element M has a higher melting point than the oxide of element K. (1 mark)
- The grid below is part of the periodic table. Use it to answer the questions that follow. The letters are not the actual symbols of the elements.
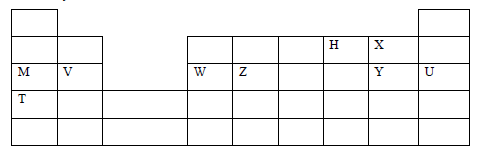
- How do the ionization energies of elements M and T compare? Explain. (1 mark)
- The chloride of element W was dissolved in water and solid sodium carbonate added to the resulting solution. Explain the observation made. (2 marks)
- the ionic radius of the ions formed by elements M and V. (2 marks)
- State one use of element T. (1 mark)
- Write a chemical equation for the reaction between element V with cold water. (1 mark)
- The table shows information about some elements (Letters are not the actual symbols of the elements)
-
- Hydrogen chloride gas was prepared and reacted with aluminium turnings as shown below.
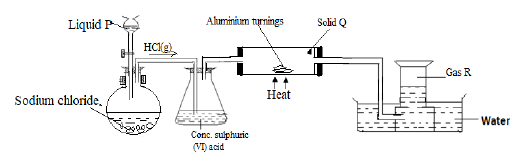
- Name liquid P. (1 mark)
- State the confirmatory test for gas R. (1 mark)
- Explain why solid Q collects further away from the heated aluminium. (1 mark)
- Sodium chloride also known as rock salt is preferred to any other chloride in the preparation of hydrogen chloride gas. Give a reason. (1 mark)
- Explain why blue litmus paper changes to red when dipped in water in the trough at the end of the experiment. (1 mark)
- A part from manufacture of hydrochloric acid, state another use of hydrogen chloride gas. (1 mark)
- Study the flow chart below.
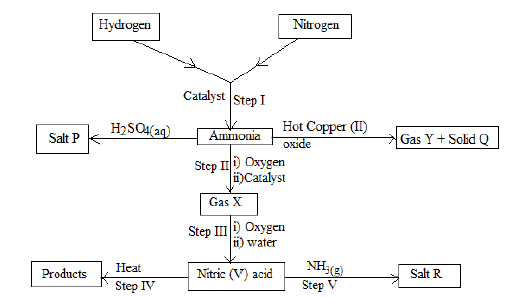
- Give one condition other than the use of the catalyst that would favour the reaction in step I. (1 mark)
- Write a chemical equation for the reaction in step III. (1 mark)
- What is the main source of hydrogen gas used in step I? (1 mark)
- Concentrated nitric (v) acid is usually transported in containers made of aluminium and not copper. Explain. (1 mark)
- Name the type of reaction that takes place when ammonia gas reacts with hot copper (II) oxide. (1 mark)
- If 200cm3 of ammonia gas measured at room temperature and pressure reacted completely with the hot copper (II) oxide, calculate the mass of solid Q formed. (Cu=64, Molar gas volume at r.t.p = 24dm3) (2 marks)
- Hydrogen chloride gas was prepared and reacted with aluminium turnings as shown below.
-
- The flow diagram below is a summary of the process involved in the preparation of a detergent. Study it and answer the questions that follow.
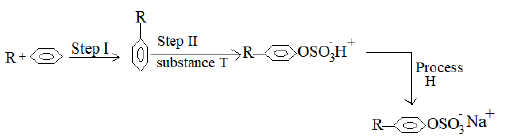
What is a detergent. (1 mark) -
- Give the IUPAC name of the starting material, substance R reacted with benzene. (1 mark)
- Identify substance T used in step II. (1 mark)
- Name process H. (1 mark)
- Give the condition necessary for the reaction in step I to occur. (1 mark)
- Write the equation for the reaction which takes place when the detergent prepared above is added to water drawn from a borehole. (1 mark)
- Give one advantage that the detergent prepared above has over soapy detergent. (1 mark)
- The general structure of the detergent prepared above is:
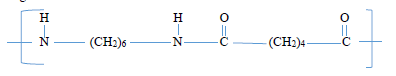
Describe how the detergent removes dirt from a garment stained with grease. (2 marks) - Nylon 6,6 is a polymer formed when two different monomers M and N are made to join forming the long chain molecule shown below with the loss of water molecules.
- Name the process that leads to the formation of the above structure. (1 mark)
- Draw the structural formula of the monomer M and N from which the above polymer is made. (1 mark)
Monomer M Monomer N - Give one environmental problem associated with the use of the polymer shown above
- The flow diagram below is a summary of the process involved in the preparation of a detergent. Study it and answer the questions that follow.
-
- The set up below shows an electrochemical cell that can be used to convert the chemical energy of a fuel directly to electrical energy.
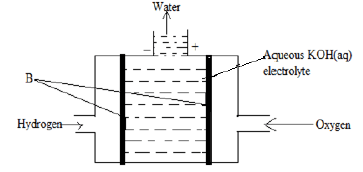
- Name the part labelled B. (1 mark)
- Write the overall cell reaction in the above hydrogen /oxygen cell. (1 mark)
- Give one of the uses of standard electrode potentials. (1 mark)
- State one advantage of secondary cells over primary cells. (1 mark)
- Below is a list of potential difference obtained when metals P, Q, R, S and T are used in the following electrochemical cell. Metal(s)/metal ions(aq)//Copper (II) ions(aq)/copper(s).
Metal E◦(volts)
P -1.10
Q -0.46
R 0.00
S +0.45
T +1.16- Identify metal R. Explain. (2 marks)
- Which two of the above metals in an electrochemical cell would produce the largest potential difference. (1 mark)
- Calculate the electromotive force of the cell chosen in (ii) above. (1 mark)
- Which of the metals above cannot be displaced by any of other metals from the solution of its ions? Explain. (2 marks)
- The set-up below was used during the electrolysis of 100cm3 of 2M copper (II) sulphate solution using inert electrodes.
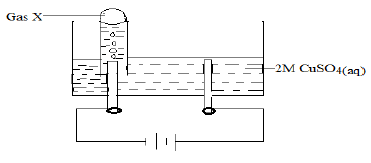
A current of 4A was passed through 100cm3 of 2M copper(II) sulphate solution for 2 hours 20 minutes. Calculate the amount of copper in the remaining solution after the experiment. (Cu=63.5, 1F=96500C) (3 marks)
- The set up below shows an electrochemical cell that can be used to convert the chemical energy of a fuel directly to electrical energy.
-
- What is meant by the term molar enthalpy of formation? (1 mark)
- Use the following standard enthalpies of combustion of graphite, hydrogen and propane.
ΔHc (graphite) = -393kJ/Mol
ΔHc (H2 (g)) = -286kJ/Mol
ΔHc (C3H8(g)) = -2219kJ/mol- Write the equation for the formation of propane. (1 mark)
- Draw an energy cycle diagram that links the heats of formation of propane with its heat of combustion and the heats of combustion of graphite and hydrogen. (2 marks)
- Calculate the standard heat of formation of propane. (2 marks)
- Propane and butane are constituents of cooking gas. Which one produces more energy per mole on combustion? Explain. (2 marks)
- Other than the enthalpy of combustion, state two factors which should be considered when choosing a fuel. (2 marks)
- The molar enthalpies of neutralization for dilute hydrochloric acid and dilute nitric(V) acid are -57.2kJ/mol while that of ethanoic acid is 55.2kJ/mol. Explain this observation. (2 marks)
- The table below shows the solubility of salt X and Y at various temperatures.
Temperature(◦C) 0 15 25 35 45 55 65 75 Solubility of X in 100g of water 12 26 38 53 72 98 124 155 Solubility of Y in 100g of water 35.0 35.8 36.2 36.6 37.0 37.4 38.0 38.0 - By using the same axes, plot graphs of solubility of salt X and Y against temperature. (3 marks)
- At what temperature are the solubilities of X and Y the same. (1 mark)
- What mass of salt Y will saturate 35g of water at 70 °C? (2 marks)
- Name the method of separation that would be used to obtain the two salts from a mixture of their solution (1 mark)
- State one application of the method named in (iv) above. (1 mark)
- The diagram below is a flow chart for the extraction of copper. Study it and answer the questions that follow.
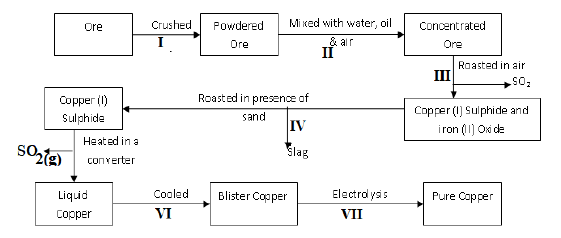
- Write the formula of the major ore of copper metal. (1 mark)
- Name process II. (1 mark)
- Give an equation for the reaction that occurs in stage III. (1 mark)
- With the aid of an equation, explain what happens in stage IV. (2 marks)
- Write half-cell equations occurring at the anode and cathode in stage VII. (2 marks)
Anode: ………………………………………………………………………………………………
Cathode: ……………………………………………..……………………………………………… - Draw a simple diagram showing the set-up that is used in electrolytic purification of copper. (2 marks)
- State one way in which environmental pollution can be prevented during extraction of copper metal. (1 mark)
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 2 Questions - Maseno Mock Examinations 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students
