Click the link below to download as a full set with all the subjects. (PDF)
https://downloads.easyelimu.com/details/35-Alliance_High_School_Pre_Trial_Examination
ALLIANCE HIGH SCHOOL
CHEMISTRY PRE-TRIAL
PAPER 1
233/1 - 2018
TIME: 2 HOURS
- A mixture consists of sulphur and iron fillings, the mixture is heated in an open crucible.
- State the observation. (1 mk)
- Write an equation for the reaction. (1 mk)
- Element K (not actual symbol of the element) has isotopes with relative abundance as shown below.
Isotope Relative Abundance (%) 105K 18.69 115K 81.28
Calculate the relative atomic mass of K. (2 mks) - The table below gives the ionization energies of the alkali metals.
Element 1stionization energy
KJmol-A 494 B 418 C 519
Which of the three metals is the least reactive. Give a reason. (1 mk) - Dry hydrogen gas was passed over heated oxide of copper weighing 14.3g, at the end of the experiment 12.7g of copper and 1.8g of water were obtained.
- Determine the formula of the oxide. (Cu - 63.5, O - 16) (2 mks)
- Name two other gases tha can be used instead of hydrogen. (1 mk)
- A piece of burning Magnesium was introduced into a gas jar of nitrogen, water was then added to the products. The resultant solution was tested with litmus paper.
- Explain the observation. (1 mk)
- Write an equation for the formation of the final solution. (1 mk)
- State one reagent that can be used to distinguish between the pairs of ions.
- Pb2+(aq) Al3+(aq) (2 mks)
- SO2-4 and SO2-3 (2 mks)
- 20cm3 of sodium carbonate solution was reacted completely with 25cm3 of a 0.8M hydrochloric acid according to the equations.
Na2CO3 + 2HCl --------> 2NaCl + CO2 + H2O
Calculate the concentrationn of sodium carbonate in grams per litre. (3 mks) - The electronic arrangement of ions of a certain element represented by letters P Q R and S
P2- 2:8:8
Q2+ 2:8
R+ 2:8
S 2:8:8- Explain why S is not represented as an ion. (1 mk)
- Which element has the largest atomic radius? Explain. (1 mk)
- A label on a bottle of Hydrochloric acid has the following information;
Density 1.134gm-3 and percentage purity 37%
Determine the molarity of the solution. (4 mks) - The following figure shows a section of apparatus used in laboratory for measuring volume.
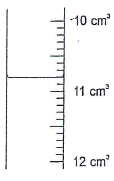
- Identify the apparatus and state the correct reading of volume. (2 mks)
- The atomic number of P and S are 6 and 17 respectively.
- Using dots and cross, draw the compound formed when P reacts with S. (1 mk)
- Name the type of bond and explain whether the compound would conduct electricity. (2 mks)
- A given volume of a gas G diffuses through a membrane in 10 seconds. Under same condition, an equal volume of oxygen diffuses for 12.5 sec. Determine the molecular mass of G. (2 mks)
- Using an equation, explain the observation made when sodium hydroxide is added to Aluminum oxide dropwise until in excess. (2 mks)
- Name the product of the reaction. (1 mk)
- Cynogen is a gaseous compound of carbon and Nitrogen only. On complete combustion in oxygen, 250cm3. Determine the formula of cynogen. (3 mks)
- A stream of Ammonia was bubbled in water containing litums papers.
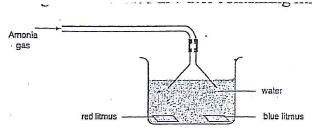
- State one physical property of the gas. (1 mk)
- Explain the observation made during the experiment. (1 mk)
- A student was given solid potassium permanganate to prepare a colourless gas.
- Draw a complete set up to carry out the experiment naming the gas. (3 mks)
- Propene and propane both decolourises bromine liquid at different conditions.
- Explain with an equation how the hydrocarbons decolourises bromine. (4 mks)
- Complete the reaction by indicating the polymer. (1 mk)
C2H4 ------> - State type of reaction and calculate the value of n given the molecular mass of polymer is 33600. (4 mks)
- In the apparatus shown in the diagram, compound M and N are reacted to produce Ammonia which is passed to T where it is burnt.
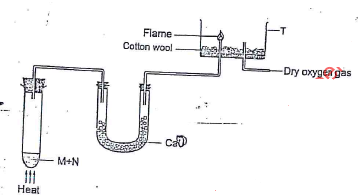
- Name the substance M and N. (2 mks)
- Role of cotton wool and calcium oxide. (1 mk)
- Write an equation for the reaction. (2 mks)
- Below is a chemical reaction.

- Using an energy level diagram, represent the reaction when vanandium(V)oxide is used. (2 mks)
- State the effect of increase in temperature to the equilibrium. (1 mk)
- Give one characterisitc of a dynamic equilibrium. (1 mk)
- Study the test below and answer the questions.
Test Observation
(i) P is heated until no
further changeA colourless liquid
condensed on the cooler
parts of the test tube
-A colourless gas which turns
Aqueous potassium chromate
(VI) green was given out and red
brown residue R was left.(ii)Chlorine gas was bubbled
through an acqueous of PSolution turn from green to yellow - Identify P and R (1 mk)
- Write an equation for test (i) (1 mk)
- Describe how a student would test for anion in solid P. (3 mks)
- Name one reagent that can be used to confirm cation in P. (1 mk)
- Name the main ores of: Iron, copper, sodium, aluminium (2 mks)
- Calculate the oxidation number of P given the following P2O5 (1 mk)
- Identify z and y given the following equation. (3 mks)
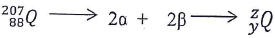
- State and explain observation made when chlorine gas is bubbled through a solution of potassium iodide. (2 mks)
- Sketch the bond formed between the complex tetramine copper (II) ions.
(1 mk) - Explain why graphite is used as a lubricant in machines. (3 mks)
- Describe how you would prepare crystals of sodium nitrate starting with 200cm3 of 2M sodium hydroxide. (3 mks)
- One liter of a gas has a mass of 1.16g at s.t.p. Analysis shows that this gas contains 92.3% carbon and 7.69 hydrogen. Calculate the molecular formular of the gas. (C = 12.0, H = 1.0) (2 mks)
- Below is a diagram of a dry cell. Study it and answer the questions that follows.
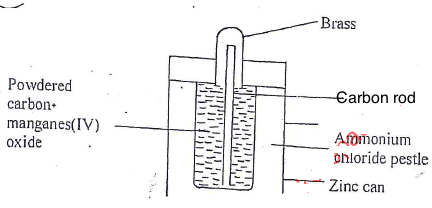
- Write an equation at anode and cathode. (2 mks)
- Why is Ammonium Chloride used as a paste rather than a dry solid. (1/2 mark)
-
- Identify the cleansing agent. (1/2 mark)
- Write the molelular formular of the cleansing agent above. (1/2 mark)
- State one advantage of using the cleansing agent. (1/2 mark)
- A student set up the electrochemical cell as represented below.
Pb(s)/Pb(NO3)(l) 1M // SnCl2(aq) (1M)/Sn(s)- State the condition under which the set up was made. (1 mk)
- Using the Eθ given below, calculate the e.m.f of the cell. (1 mk)
Pb(aq)2+ + 2e- -----> Pb(s) -- 0.13v
Sn(aq)2+ + 2e- -----> Sn(s) -- 0.14v
Download Chemistry Paper 1 - Alliance High School Pre-Trial Examination 2018.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

