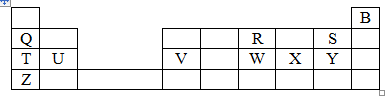
- The grid below represents part of the periodic table. Study it and answer the questions that follow. Letters are not the actual symbols of the elements.
- Select a letter that represents the most reactive non-metal. Explain (2 marks)
- Select a letter that represents an element that forms an ion with a charge of 2- (1 mark)
- Select an alkaline earth metal (1 mark)
- Which group 1 element has highest first ionization energy? Explain (2 marks)
- What is the formula of the compound formed when V reacts with X? (1 mark)
- What type of chemical bond exists in a compound formed when R and S react? (1 mark)
- Explain why the atomic radius of S is smaller than that of Q. (2 marks)
- Explain why molten calcium chloride conducts electricity while carbon tetrachloride does not conduct electricity (2 marks)
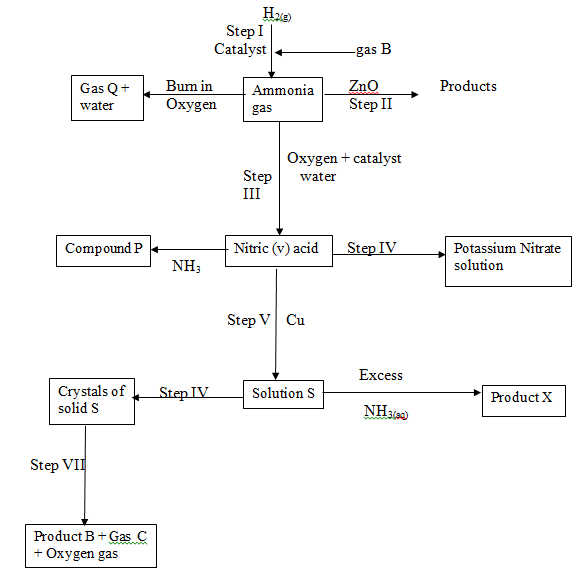
- Study the flow chart below and answer questions that follow:
- State one source of gas B (1 mark)
- Name the catalysts used in; (2 marks)
- Step I
- Step III
- Write chemical equations for reactions in; (2 marks)
- Step II
- Step VII
- Name the process that takes place in; (2 marks)
- Step IV
- Step VI
- Name and write the chemical formula of product X
Name (1 mark)
Formula (1 mark) - State one laboratory use of compound P (1 mark)
- Identify any other gas that can be used instead of Ammonia in step II (1 mark)
- State one use of gas Q (1 mark)
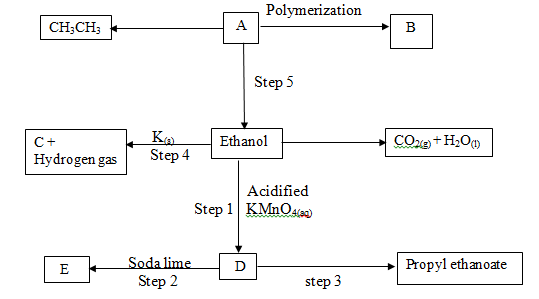
-
- Give the systematic names of the following compounds (2 marks)
- CH3CH2OK
- CH3CH2COOCH2CH3
- Describe a chemical test other than burning that can be used to distinguish between ethene and ethane (2 marks)
- Study the flow chart below and answer the questions that follow.
- Name compounds C and E (1 mark)
- Write the equation for the reaction in (2 marks)
Step I
Step 3 - Give the reagents for the reaction in step 5 to occur. (2 marks)
- Calculate the mass of ethanol which when burnt in air at room temperature and pressure would produce 10dm3 of carbon (IV) oxide gas
(C = 12.0, O = 16.0, Molar gas volume = 24dm3) (3 marks)
- Give the systematic names of the following compounds (2 marks)
- In an experiment to determine the molar heat of neutralization of hydrochloric acid with sodium hydroxide, students of a secondary school reacted 100cm3 of 1.0M hydrochloric acid with 50cm3 of 2M sodium hydroxide and obtained the following results.
Initial temperature of acid = 25.0°C
Initial temperature of alkali = 25.5°C
Highest tempature reached with acid and alkali mixture = 34.0°C- Define the term molar heat of neutralization. (1 mark)
- Write an ionic equation of the reaction between hydrochloric acid and sodium hydroxide.(1 mark)
- Calculate:
- The change in temperature. (1 mark)
- The amount of heat of neutralization of sodium hydroxide. (Specific heat capacity=4.2 kJ/kg/K) (1 mark)
- Write the thermochemical equation for the reaction. (1 mark)
- Draw an energy level diagram for the reaction. (2 marks)
- Use the information given below to answer the questions that follow.
2H2(g) + O2(g) →2H2O(l) ΔHθ = -572KJmol-1
2H2O2(l) →2H2O(l) + O2(g) ΔHθ = -196KJmol-1- Calculate the standard enthalpy of formation of Hydrogen peroxide (2 marks)
- Draw an energy cycle diagram for the information above linking it with the enthalpy of formation of Hydrogen peroxide. (2 marks)
- State two applications of enthalpy of combustion (1 mark)
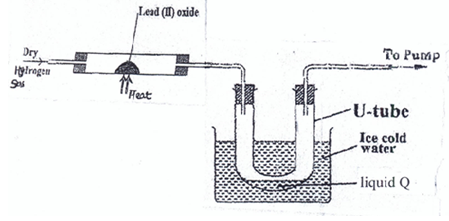
- Study the diagram below and answer the questions that follow.
-
- State and explain the observation(s) made in the combustion at the end of the experiment
Observation(1 mark)
Explanation (1 mark) - Identify a metal oxide that can be used in place of lead (II) oxide (½ mark)
- State and explain the observation(s) made in the combustion at the end of the experiment
- Write the balanced chemical equation for the reaction in the combustion tube (1 mark)
- Give one chemical test for liquid Q (1 mark)
- Explain what would happen if magnesium oxide was used instead of lead (II) oxide in the experiment (1½ mks)
State one observation made when a small piece of potassium metal is dropped into a trough containing liquid Q(1 mark) - Name any one source of Hydrogen gas. (1 mark)
-
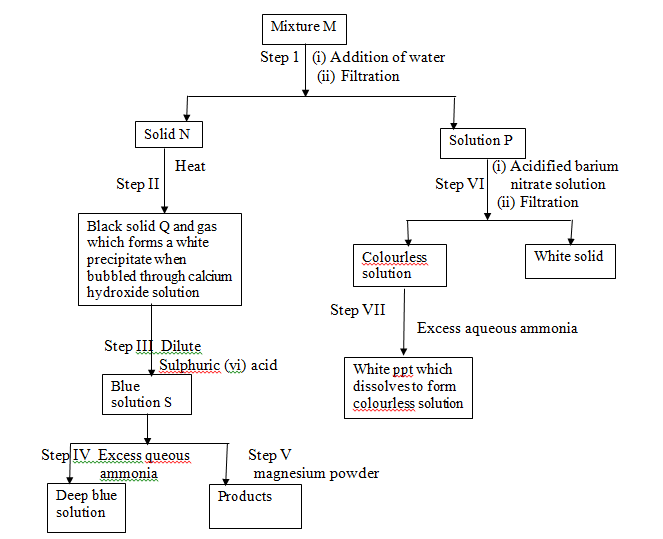
- The flow chart below shows a sequence of reactions involving a mixture of two salts, mixture M. Study it and answer the questions that follow.
- Write the formula of the following;
- Anion in solid N (1 mark)
- The two salts present in mixture M (1 mark)
- Write the ionic equation for the reaction in step VI (1 mark)
- State the observations made in step V (1 mark)
- In an experiment to determine solubility of potassium chlorate, 5g of potassium chlorate (KClO3) was dissolved in water and solubility of the salt was determined at various temperatures as shown in the table.
Volume of distilled water (cm3)
Temperature at which 1st crystal appears (°C)
Solubility of solid of (g/100g of water)
4
68.0
6
58.5
8
53.0
10
47.5
12
35.0
- Define the term solubility (1 mark)
- Complete the table by calculating the solubility of potassium chlorate (2½ marks
- Plot a graph of solubility of potassium chlorate (g/100g of water) against temperature (°C) (3 marks)
- State the relationship between solubility of potassium chlorate and temperature (1 mark)
- Use the graph to determine:
- The solubility of potassium chlorate at 50°C (½ mark)
- The molarity of the solution at temperature stated in e(i) above (2 marks)
(K = 39, Cl = 35.5, O = 16)
- Write the formula of the following;
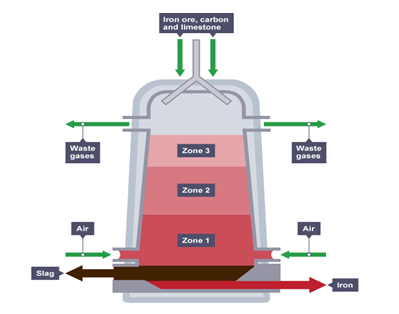
- The diagram below represent the blast furnace for extraction of iron metal. Study it and answer the questions that follow.
- The chief ore is Haematite. Name one other ore used in extraction of iron (1 mark)
- In the blast furnace carbon (II) oxide is used as a reducing agent. Write an equation for the reaction between carbon (II) oxide and oxide of iron (1 mark)
- Silica impurities react with calcium oxide from the kiln.
- Write an equation for the reaction (1 mark)
- Explain why it is possible for the reaction to occur. (1 mark)
- Explain why iron is a good conductor of electricity. (1 mark)
- Iron is malleable and ductile. Give the difference between malleability and ductility(2 marks)
Malleability
Ductility - Extraction of iron is cheaper than extraction of aluminium. Explain (2 marks)
- Identify one waste gas from the furnace and state its effect on the environment (2 marks)
- State one use of steel in construction industry (1 mark)
Download CHEMISTRY PAPER 2 - KCSE 2019 MOKASA PRE MOCK EXAMINATION.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students