- State the uses of the following laboratory apparatus ( 1mk)
- Tongs
- Forceps (1mk
- Give an example of adsorbent material used in chromatography in the laboratory (1mk)
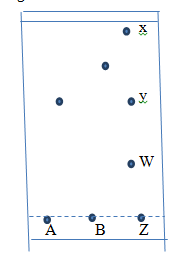
- Spots of pure pigments A and B and a mixture Z were placed or a filter paper and allowed to dry. The paper was then dipped in a solvent. The results obtained were as on the papers chromatogram.
- Why did substance X move furthest ? (2mks)
- Which substance was not a component of Z ? (1mk)
-
- What is etching of metals insofar as uses of acid is concerned? (1mk)
- Name two acids that are used as foodflavours (2mks)
-
- Statethe necessary connections for the corrosion of iron metal (1mk)
- State the importance of corrosion of metals (2mks)
- An unknown gas takes 56 seconds to diffuse through a tiny orifice. An equal volume of oxygen measured at the same temperature and pressure takes 30 seconds to diffuse through the same orifice .Calculate the molecular mass of the unknown gas. (3mks)
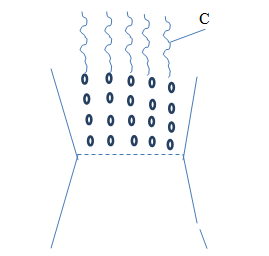
- The diagram below shows the burning charcoal stove. Use it to answer thequestions that follow:
- Whatcolour is section C. (1mk)
- Write an equation for the reaction taking place in section C. (1mk)
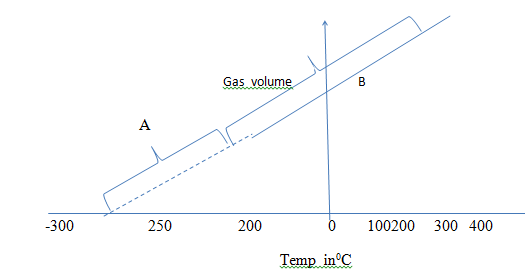
- Study the graph below and answer the questions that follow: -
- Whose law is represented by the graph (1mks)
- What do regions A and B represent (2mks)
- Use the following bond energies to answer the questions that follow:-
Bond Energy in K Jmol-1
H- H +436
Cl- Cl +244
H - Cl +432
Determine if the reaction below is exothermic or endothermic
H 2(g) + Cl2(g) →2HCl (g) (3mks) - Study the table below and answer the questions that follow:-
Element
Ionic formula
Electron arrangement
Atomic radi mm
Ionic radi mm
A
A2+
2.8
0.136
0.65
B
B-
2.8.8
0.099
0.181
C
C-+
2.8.8
9.208
0.133
D
D2+
2.8.8
0.174
0.099
- Which elements have similar chemical properties .Explain (2mks)
- Explain the trend of the atomic radii to the ionic of element B (2mks)
-
- Name the two types of bonds common in the following compounds ,carbon(ii) oxide and hydrozoniumion. (2mks)
- Explain how each bond is formed (2mks)
- Whenanhydrous calcium chlorideis exposed to the atmosphere the following reaction takes place as per the equation below:-
Cacl2(s) →H2O→CaCl2(aq)- Name the process that took place (1mk)
- State one use of the property displayed by the anhydrous calcium chloride (1mk)
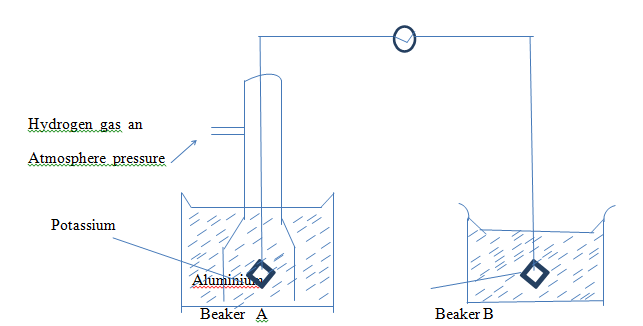
- The diagram below represents part of the apparatus to be used for the determination of the standard electrode potential E® of aluminiumAl3+(aq)/Al (s)
- Whatis missing in the diagram and state its function (1 ½ mk)
- Name the solution which could be placed in beaker A and B and state their concentration (2mks)
- Complete the diagram (1/2 mk)
- Complete the following nuclear equations and identify nuclideA and B
- 24 Na A + O e (1mk)
11 -1 - 14 C B + O (1mk)
6 -1 - Distinguish between nuclear fission and nuclearfusion (2mks)
- 24 Na A + O e (1mk)
- Explain how the following factors affect the rate of a chemical reaction
- Temperature (2mks)
- Concentration (2mks)
-
- In which homologous series do the following compounds belong
- CH3CCH ( ½mk)
- CH3COOCH3 ( ½mk)
- Name two reagents that reacted to form the compound in (ii) above (1mk)
- In which homologous series do the following compounds belong
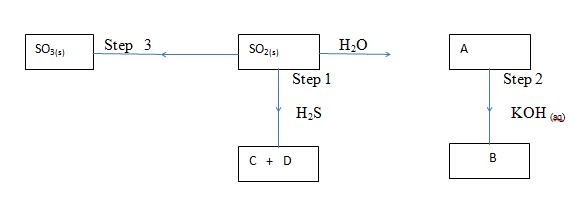
- Study the flow chart below and answer the questions that follow:-
- Write an equation for the formation of
- A (1mk)
- B (1mk)
- State the property of SO2 exhibited in step 1 (1mk)
- Write an equation for the formation of
- A label on a bottle containingsulphuric (vi) acid has the following information
Density = 1.836g/cm3
Percentage purity = 98%
Relative formula mass = 98- Calculate the concentration of the acid in moles per litre (2mks)
- The volume of the acid that should be diluted to produce 500cm3 of 1m sulphuric (vi) acid (1mk)
- You are provided with dilute sulphuric (vi) acid. 2m ammonia solution , other laboratory apparatus and an ore you suspect to contain zinc. Explain briefly how you would confirm sthe presence of zinc in the ore. (3mks)
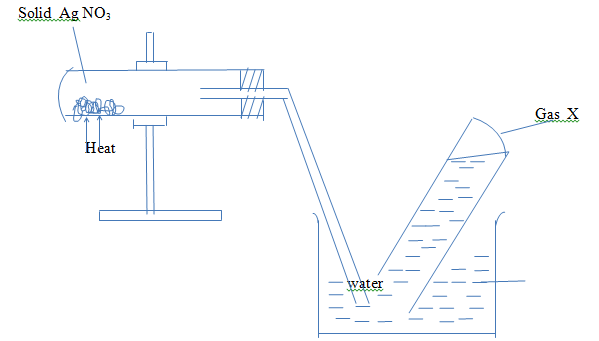
- A studentset – up the apparatus below in an attempt to prepare and collect nitrogen (iv) oxide gas.Study the set –up and answer the questions that follow:-
- Identify gas X (1mk)
- Explain why nitrogen (iv) oxide was not collected (2mks)
- Write the equation for the reaction that occurred (1mk )
-
- Chlorine is used to treat water in drinking water supply plants. Explain how the micro - organisms are eradicated (2mks)
- Where else can the property of chlorine above be applied (1mk)
-
- In the laboratory preparation of hydrogen gas nitric acid is not used .Explain (1mk)
- Givetwo examples of compounds found naturally that contain hydrogen (1mk)
-
- When 100cm3 of 1m of hydrochloric acid was neutralized by an equal volume of 1m sodium hydroxide solution, both initially at 200c, the temperature of the mixture rose to a maximum of 2 6.9 0C what is the molar enthalpy of neutralization of sodium hydroxide? (Specific heat capacity of each solution is 4.2 Jg-1k - 1 ) (3mks)
- Write the thermochemical equation for the reaction (1mk)
- Copper -64 has a half life of 12.8 days.
- What percentage of copper 64 will remain after 38.4days (2mks)
- Givetwo uses of radioactive isotopes in medicine (2mks
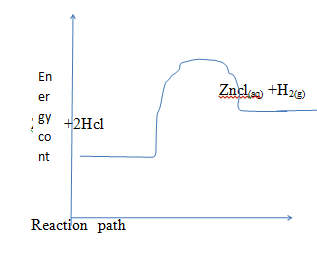
- The diagram below shows an energy level diagram for the reaction
Zn (g) +2HCl (aq)→ZnCl2(aq) + H2(g)- State the type of reaction represented and Give a reason (1mk)
- Showthe activation energy in the diagram (1mk
- A current of 4 amperes was passed through 100cm3 of 1m copper ( ii) sulphatesolution for 1 hr 10minutes .Calculate the amount of copper in the remaining solution after the experiment(F = 96500 Cu = 63.5 ) (3mks
- Explain why papers bleached using sulphur (iv) oxide turn brown when exposed to the atmosphere (1mk)
Download CHEMISTRY PAPER 1 - KCSE 2019 JOINT PRE MOCK EXAMINATION NAMBALE.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students